Therapeutic agent for tumors identified by phosphorylation of proto-oncogene protein belonging to vav family
A proto-oncogene and therapeutic agent technology applied to the field of therapeutic agents for tumors identified through the phosphorylation of proto-oncogene proteins belonging to the VAV family can solve problems such as clinical development and achieve high effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
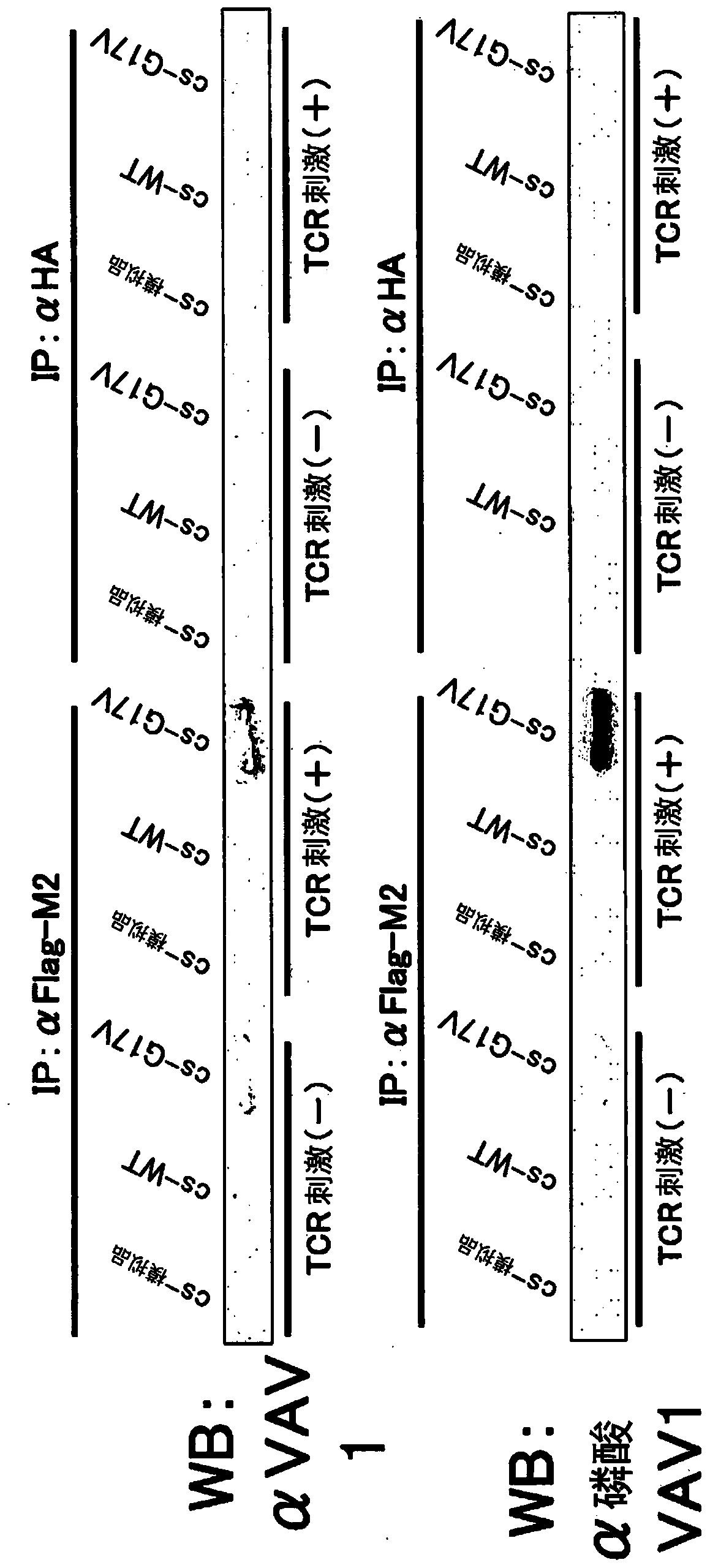
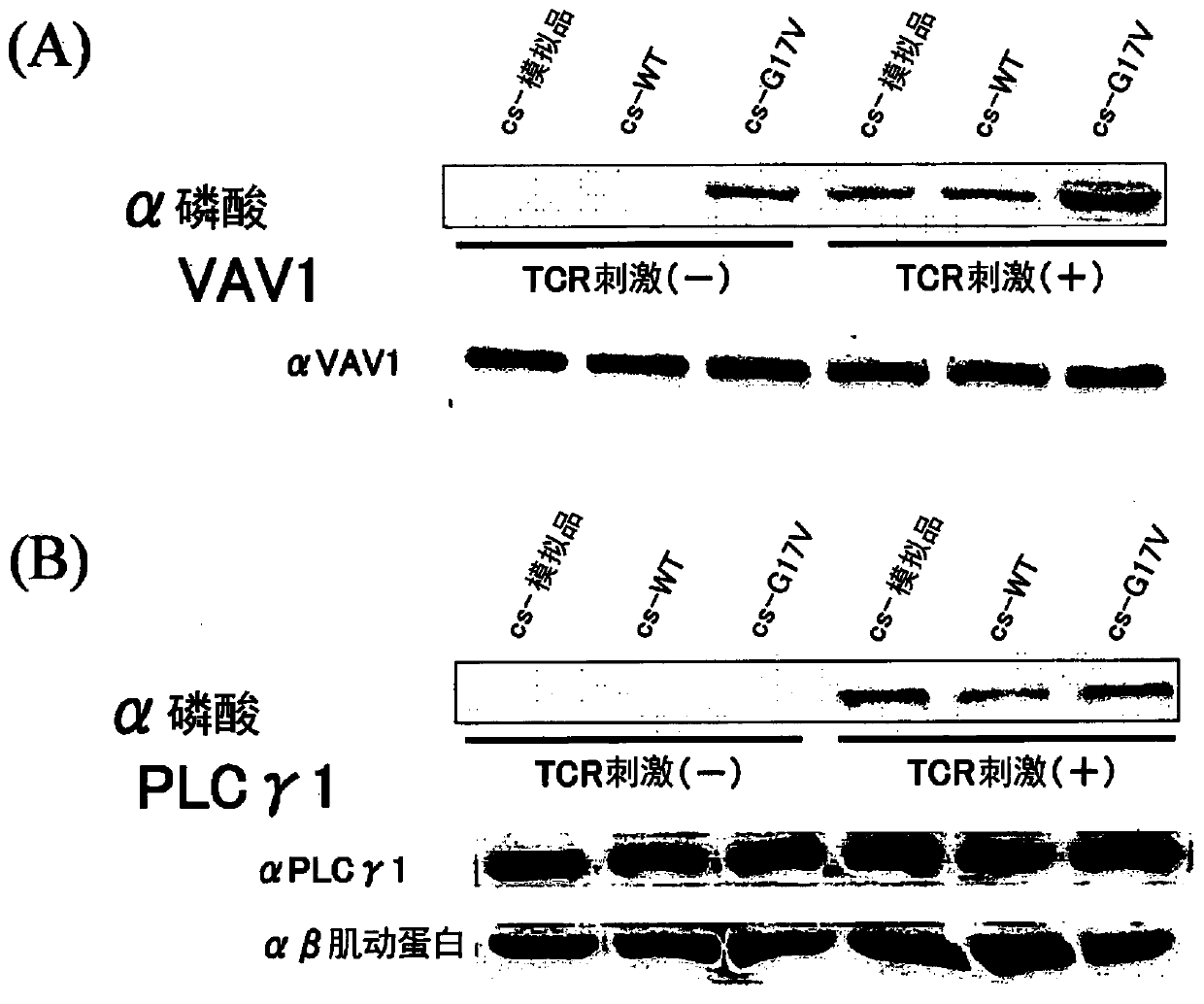
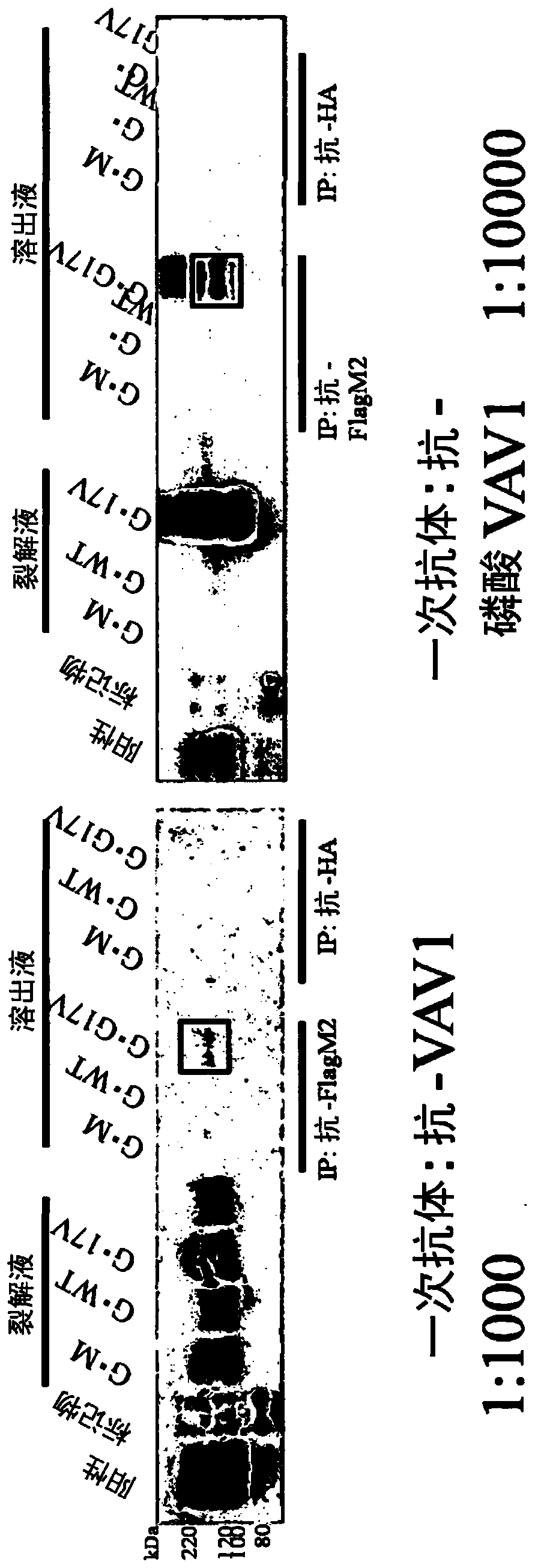
[0131] (1) Jurkat cells (T cells) expressing wild-type RHOA cDNA by TET-ON or the coding region of RHOA (hereinafter referred to as G17V RHOA cDNA. Leukemia cell line) each 2×10 5 / mL was inoculated into 3 15 cm petri dishes in RPMI (10% FCS, 1% PS). Add doxycycline in such a way that the final concentration is 2 μg / mL. The next day, the cells were recovered temporarily, and centrifuged at 6×10 5 / mL each was inoculated into 3 15 cm Petri dishes in RPMI (serum-free). After 4 hours, the cells were recovered, sterilized, washed once with phosphate-buffered saline (PBS), and adjusted to 2×10 7 / ml, transferred to a 15ml tube, and incubated at 37°C for 5 minutes. Join LEAF TM Purify anti-human CD3 Ab (BioLegend), anti-mouse IgG antibody Ab (each added in an amount of 2 μg / ml). Incubate at 37 °C for 5 min or 30 min. Add 10ml of cooled PBS, centrifuge, and remove the supernatant. Lysis buffer (with complete protease inhibitors and PhosSTOP added beforehand) was added at 1000...
Embodiment 2
[0137] Using lentivirus, the SU9T01 cells (adult T-cell leukemia / lymphoma cell lines) expressing wild-type RHOA or G17V RHOA cDNA through TET-ON were divided into 2×10 5 / mL each was inoculated into 3 15 cm Petri dishes in RPMI (10% FCS, 1% PS). Add doxycycline in such a way that the final concentration is 2 μg / mL. After the cells were recovered the next day, they were washed once with PBS. Add 1000 μl / tube of lysis buffer to the pellet. Thereafter, experiments were performed in the same manner as the Jurkat cells of Example 1. show the result in image 3 , 4 .
Embodiment 3
[0139] LEAF was diluted with sterile phosphate-buffered saline (PBS) TM Purified anti-human CD3 Ab (BioLegend) was adjusted to 10 μg / ml, and 50 μL / well was dispensed into each 96-well flat-bottom plate, and incubated overnight at 4°C. After removing the antibody solution, wash 3 times with sterilized PBS.
[0140] Take 5×10 4 Seed Jukat cells per well into 24-well plates. The next day, X-tremeGENE HP DNA transfection reagent was used to transiently transfect the pGL4.30 vector (Promega) inserted with the NFAT response sequence and the cDNA encoding firefly luciferase, and the pGL4.30 vector (Promega) inserted with the cDNA encoding Renilla luciferase. phRL vector (Promega), pEF vector inserted with wild-type or G17VRHOA cDNA. 24 hours after transfection, the cultured cell suspension was dispensed at 150 μl / well.
[0141] After 7 hours, the cultured cell solution was recovered into the tube, and 200 μl / well of PBS was further dispensed into the culture plate, transferred to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com