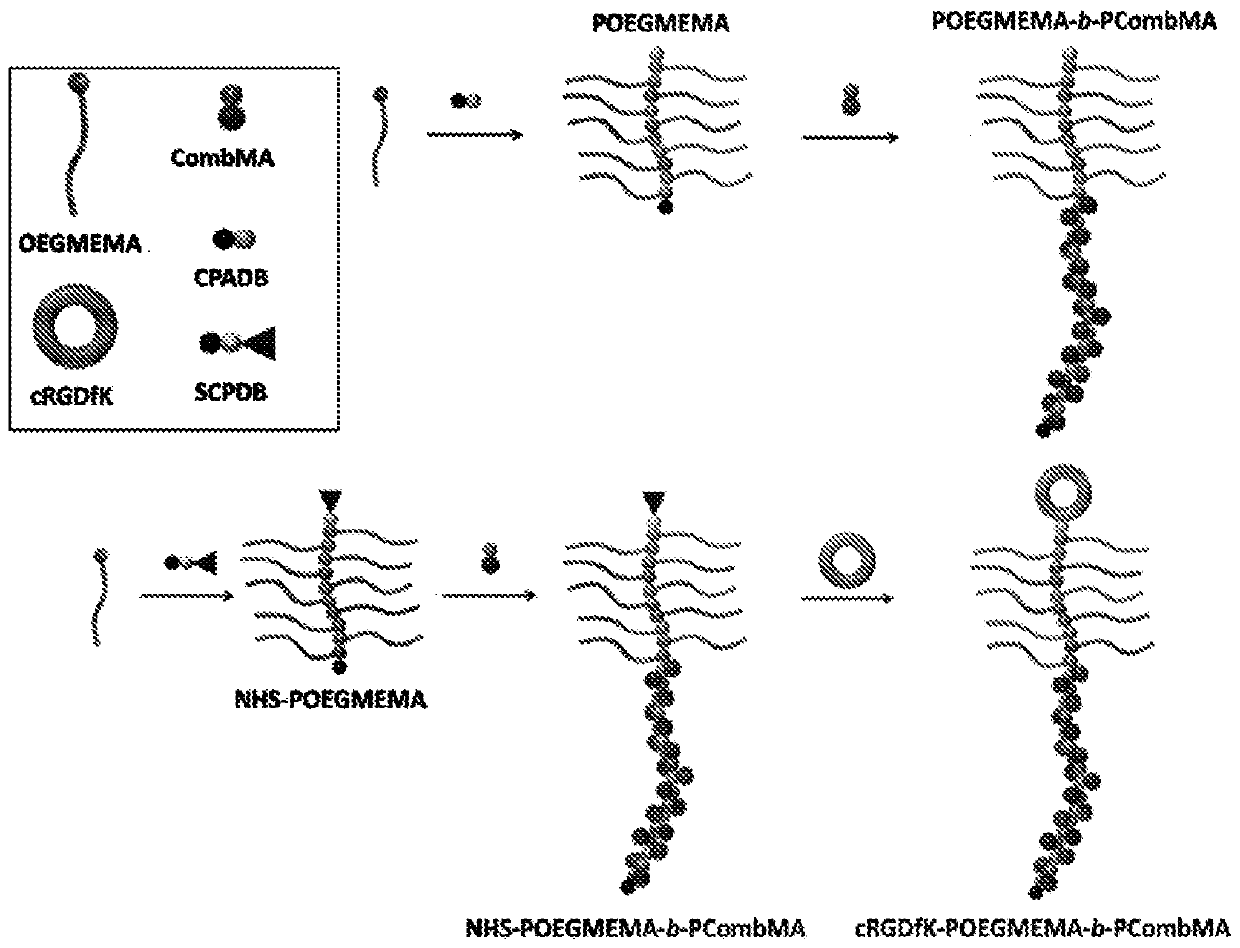
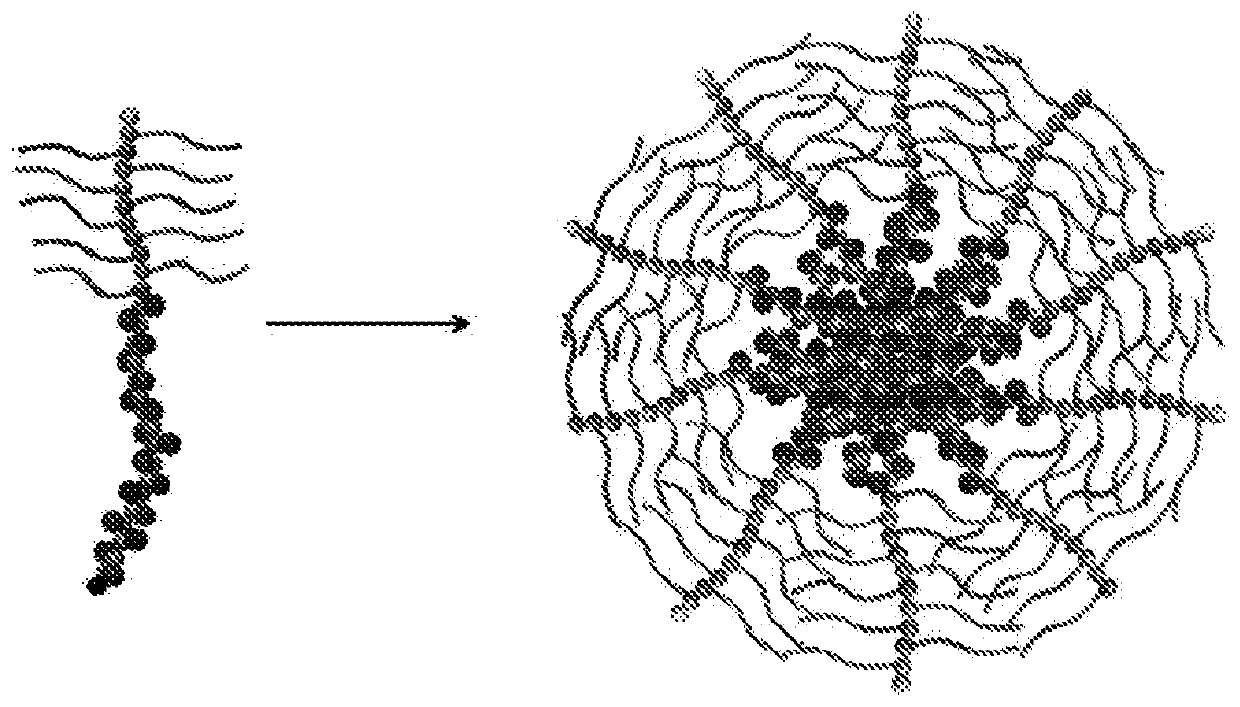
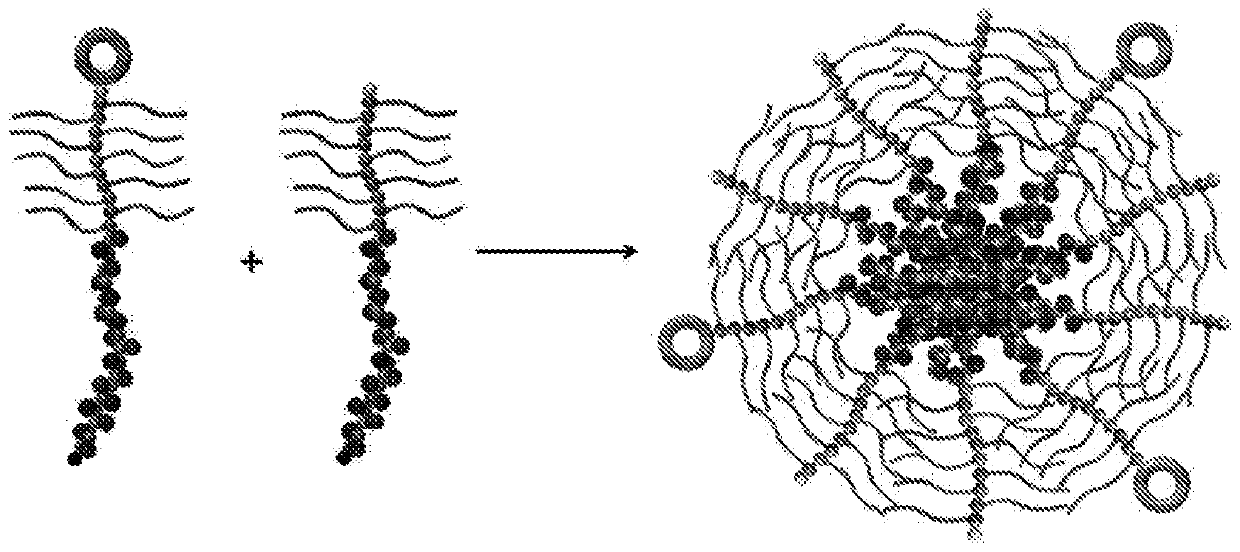
Self-assembled diblock copolymers composed of pegmema and drug bearing polymeric segments
A technology of block copolymers and polymers, used in anticancer agents for cancer treatment, 3] In order to solve the above problems, in the field of treatment of diseases, it can solve problems such as unclear structures and unexpected biological interactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
[0222] Example 1A: Synthesis of polymerizable Compretin-A4 monomer (CombMA)
[0223] In the case of N2, compretidine-A4 (300mg, 0.95mmol), triethylamine (TEA, 191mg, 1.89mmol), methacryloyl chloride (198mg, 1.89mmol) were dissolved in a 25mL round bottom flask Dry in dichloromethane (DCM, 10 mL). The reaction was stirred at room temperature for 16 hours. Use saturated NaHCO 3 (20mL x 2) and distilled water (20mL x 2) to extract the crude product. Na for organic layer 2 SO 4 Drying is performed and the solvent is evaporated. CombMA monomer was purified using column chromatography on silica with hexane.
Embodiment 1B
[0224] Example 1B: Preparation of Chain Transfer Agents (CTAs) with Reactive Functional Groups
[0225] 4-cyanopentanoic acid dithiovalerate (CPDB) is the CTA, and this CTA is modified with N-hydroxysuccinimide (NHS) according to the following procedure, NHS being the reactive functional group;
[0226] Briefly, CPADB (200 mg, 0.72 mmol) and N-hydroxysuccinimide (125 mg, 1.07 mmol) were dissolved in anhydrous DCM (4 mL). Dicyclohexylcarbodiimide (DCC) (177 mg, 0.86 mmol) was dissolved in anhydrous DCM (1 mL). The two solution mixtures were then combined, and the reaction mixture was stirred at room temperature in the dark for 16 hours. The insoluble white by-product dicyclohexylurea (DCU) was removed by filtration. The obtained solution was dried under vacuum and the crude product was purified by column chromatography on silica with hexane and EtOAC.
Embodiment 1C
[0227] Example 1C: Synthesis of POEGMEMA and NHS-POEGMEMA Homopolymers
[0228] Reversible addition-fragmentation chain transfer (RAFT) was used to synthesize POEGMEMA and NHS-activated POEGMEMA (NHS-POEGMEMA) homopolymers. To synthesize POEGMEMA polymer, to a solution of OEGMA (600 mg, 2.0 mmol) and CPADB (20.12 mg, 0.072 mmol) in DMF (3 mL) was added AIBN (1.31 mg, 0.008 mmol). use N 2 Sweep the mixture to remove O 2 and polymerized with stirring at 70°C. Polymerization was stopped by cooling and air exposure. POEGMEMA polymer was purified by precipitation in diethyl ether. The polymer precipitate was dried under vacuum to afford about 350 EGMEMA repeating units (460 mg, 77% yield). NHS-POEGMEMA polymer was synthesized using the same procedure using SCPDB (27.10 mg, 0.072 mmol) as a chain transfer agent to provide approximately 35 OEGMEMA repeating units (480 mg, 80% yield).
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com