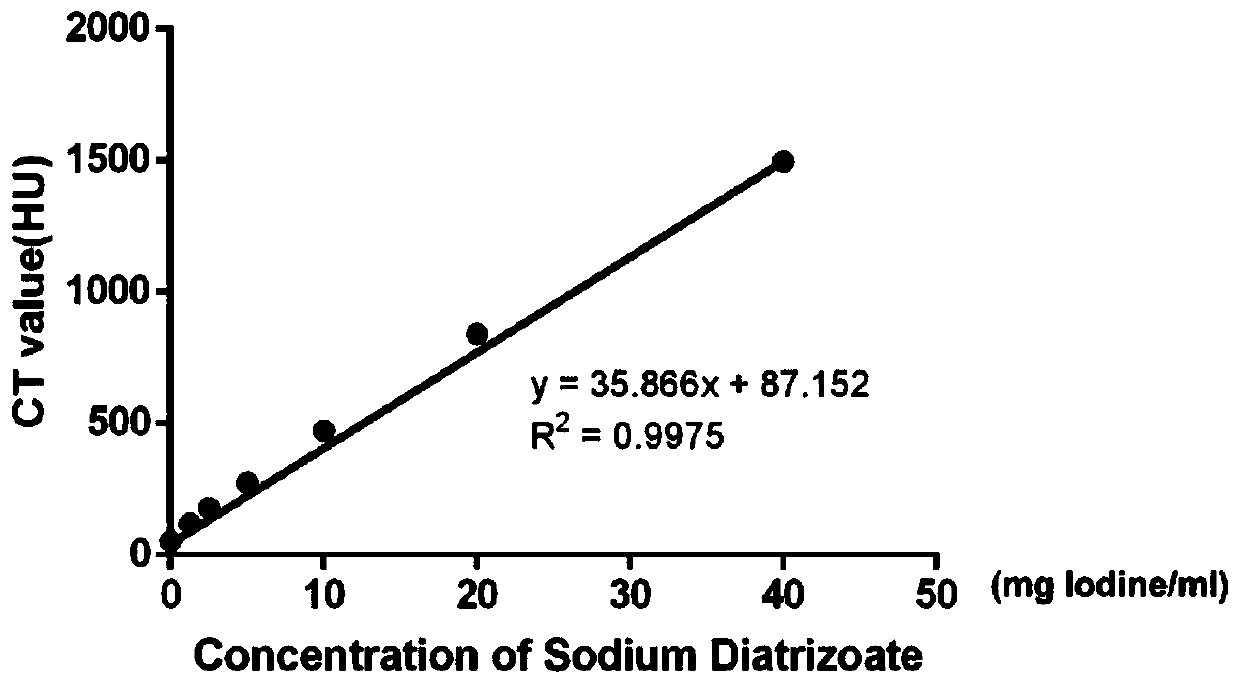
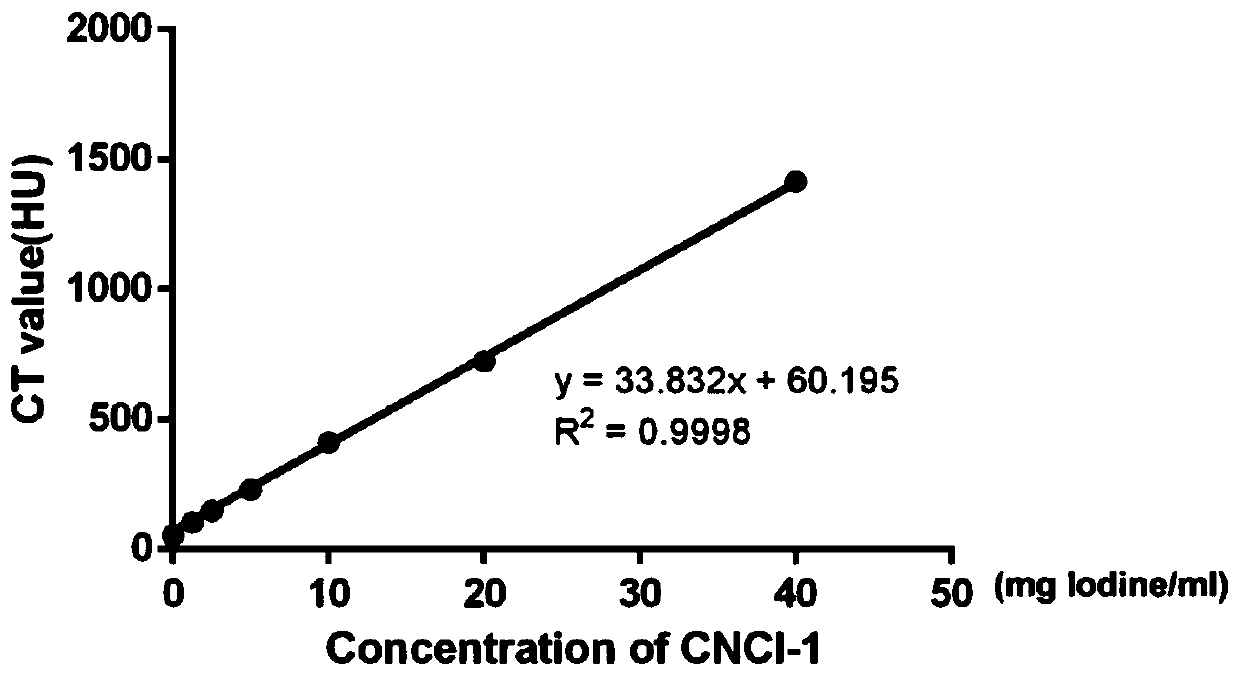
Novel bimodal micromolecular contrast agent and preparation method and application thereof
A dual-modality, contrast agent technology, used in X-ray contrast agent preparation, general/multifunctional contrast agents, pharmaceutical formulations, etc., can solve problems such as inability to identify liver tumors and weak penetration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Preparation of compound CNCI-1, the structural formula is as follows:
[0090]
[0091]Step 1: Put 4g 4-hydrazinobenzoic acid, 3.96mL 3-methyl-2-butanone, 4.32g sodium acetate, and 60mL acetic acid into a 250mL three-neck flask, under nitrogen protection; stir at 25°C for 3h, and then react at 120°C 7h; after the reaction, the mixture was transferred to dichloromethane (DCM) for extraction with water, and the organic phases were combined and concentrated; separated by column chromatography (DCM:methanol=50:1), and concentrated to obtain yellow solid compound 2 with a yield of 61%.
[0092] 1 H NMR (300MHz, CDCl 3 )δ(ppm): 8.17(d, J=8.19Hz, 1H), 8.08(s, 1H), 7.67(d, J=8.19Hz, 1H), 2.41(s, 3H, ), 1.40(s, 6H ).
[0093] Step 2: Put 4g of compound 2, 11.93mL of 1,4-butanesultone, 50mL of o-dichlorobenzene into a 250mL three-neck flask in sequence, under nitrogen protection, and refluxed at 180°C for 9h; After washing with acetone three times, a pink solid compound 3 ...
Embodiment 2
[0110] Preparation of compound CNCI-2, the structural formula is as follows:
[0111]
[0112] The preparation method is the same as that of Example 1, except that 1,3-propanediamine is used instead of ethylenediamine for the reaction in step 4, and the rest of the synthesis steps remain unchanged to obtain the final product compound CNCI-2 with a yield of 39%.
[0113] 1 H NMR (300MHz, DMSO) δ (ppm): 9.95 (m, 4H), 8.63-8.46 (m, 4H), 8.03 (s, 2H), 8.03-7.88 (m, 5H), 7.48 (d, J= 8.13Hz, 2H), 6.66-6.48(m, 4H), 4.11(br, 4H), 3.40(m, 8H), 2.53(m, 4H), 2.01(s, 12H), 1.85(m, 4H), 1.74(m, 8H), 1.66(s, 12H).
Embodiment 3
[0115] Preparation of compound CNCI-3, the structural formula is as follows:
[0116]
[0117] The preparation method is the same as that of Example 1, except that 1,4-butanediamine is used instead of ethylenediamine for the reaction in step 4, and the rest of the synthesis steps remain unchanged to obtain the final product compound CNCI-3 with a yield of 30%.
[0118] 1 H NMR (300MHz, DMSO) δ (ppm): 9.95 (m, 4H), 8.63-8.46 (m, 4H), 8.02-7.87 (m, 7H), 7.47 (d, J=7.65Hz, 2H), 6.62 -6.49(m,4H),4.11(br,4H),3.42(m,8H),2.54(m,4H),2.01(s,12H),1.92-1.73(m,16H),1.66(s,12H) ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com