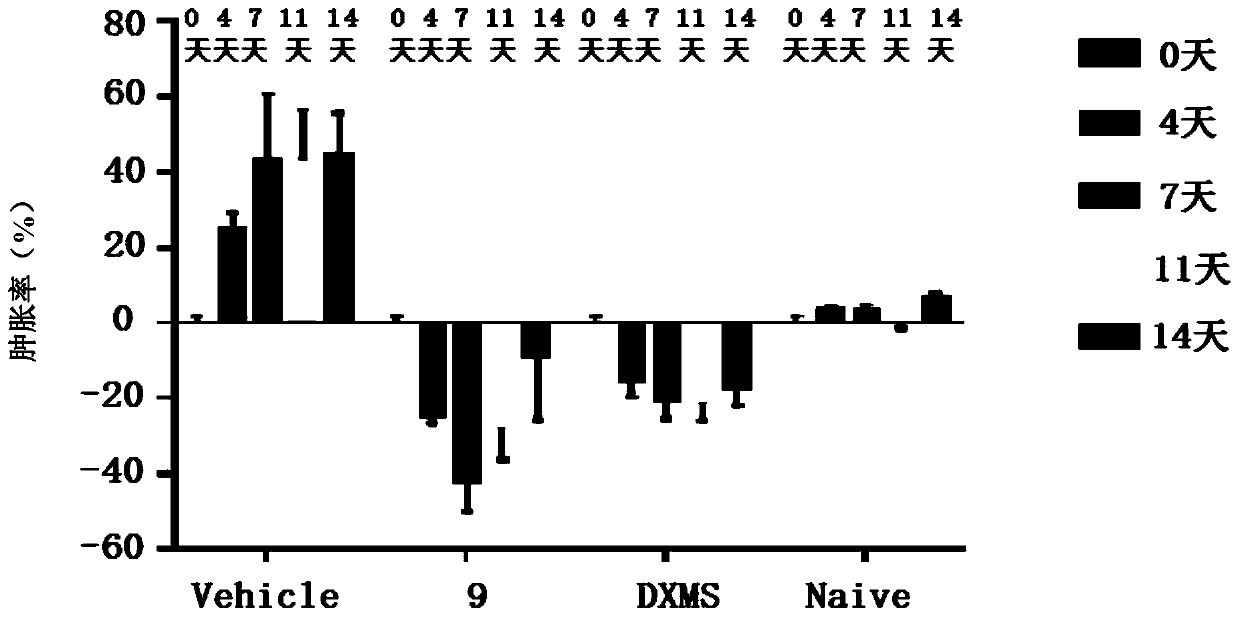
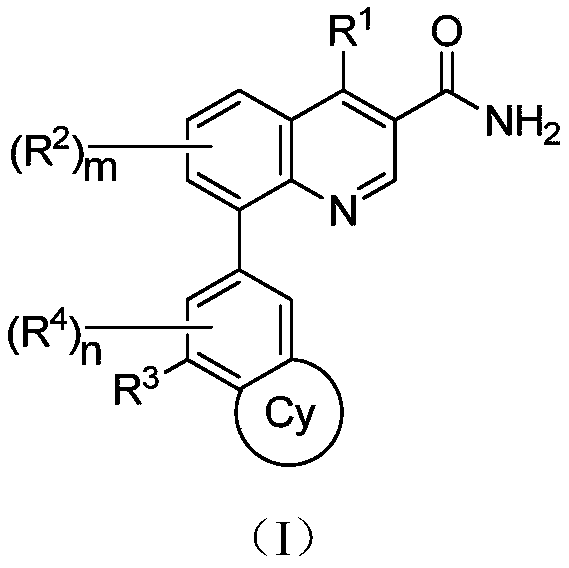
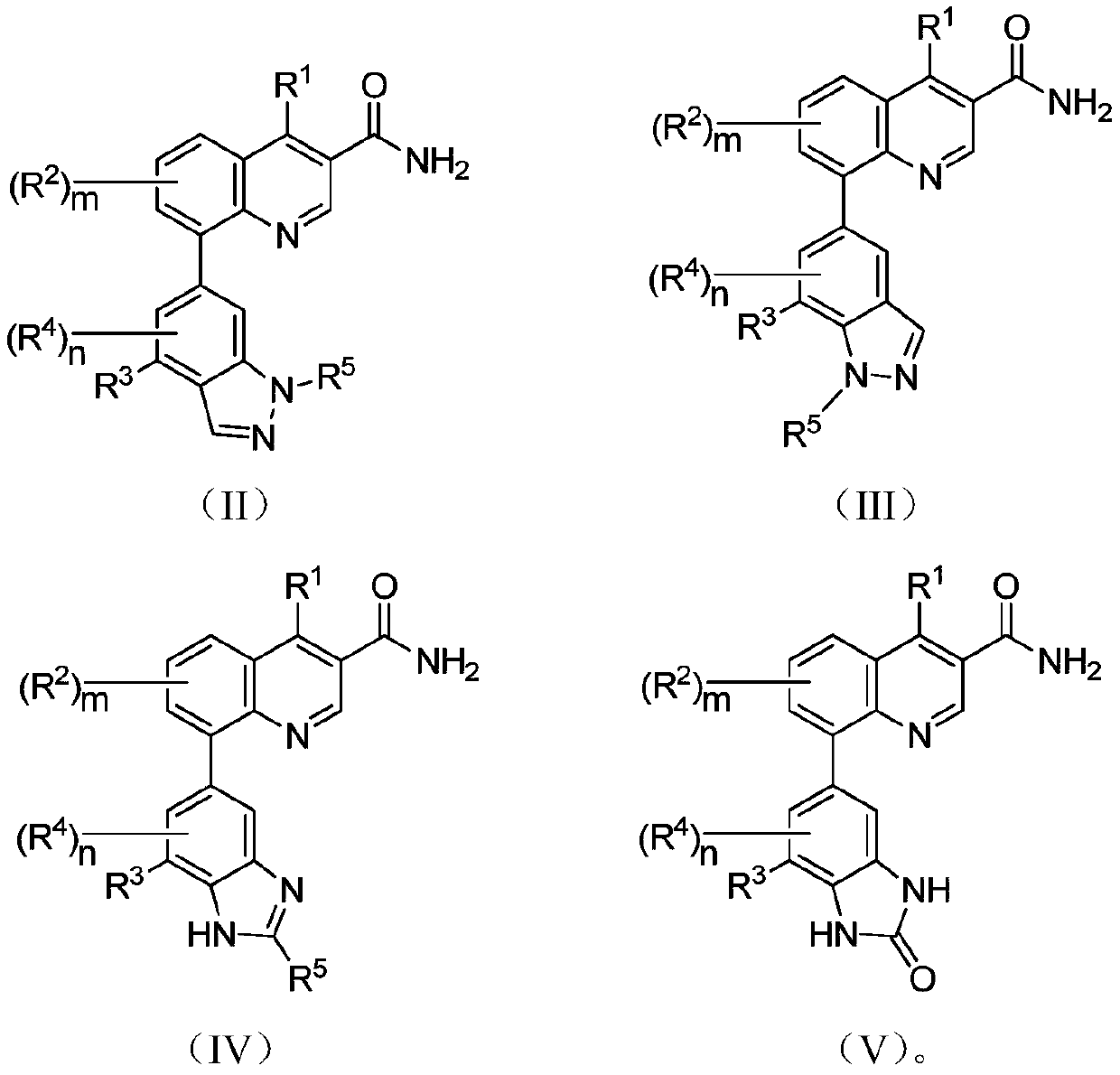
Micromolecular reversible BTK inhibitor for treating rheumatoid arthritis
A technology of solvates and compounds, which is applied in the field of small molecule reversible BTK inhibitors, pharmaceutical compositions, compounds, and the preparation of medicines, and can solve problems such as no cure for RA.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0097] The preparation of compound shown in formula (I)
[0098] Multi-substituted quinoline compounds shown in formula (I) can be prepared by a method comprising the following steps:
[0099] The quinoline compound shown in formula A1 and the substituted boronic acid or boronic acid ester shown in formula A2 undergo Suzuki coupling reaction to obtain the multi-substituted quinoline compound shown in formula (I);
[0100]
[0101] In formula A1, X is bromine, chlorine, iodine or trifluorosulfonic acid group; R 1 , R 2 and m are defined the same as previously described;
[0102] In formula A2, Y is boric acid or boric acid ester; R 3 , R 4 The definitions of , Cy and n are the same as described above.
[0103] In the above-mentioned preparation method, the conditions of the Suzuki coupling reaction are as follows:
[0104] The catalyst is Pd(dppf)Cl 2 ; The base is anhydrous sodium carbonate; the solvent is 1,4-dioxane; the temperature is 140° C.; the time is 1 to 4 h...
Embodiment 1
[0218] The preparation of compound shown in embodiment 1, formula 1
[0219]
[0220] 4-Amino-8-bromoquinoline-3-carboxamide (26.6mg, 0.1mmol), 6-indazole boronic acid (21mg, 0.13mmol) and 2M sodium carbonate solution (0.2mL, 0.4mmol) were dissolved in 2.5mL 1 , 4-dioxane, and finally [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium dichloromethane complex (8.0mg, 0.01mmol) was added. React in a microwave reactor at 140°C for 1 hour. The residual solvent was removed under reduced pressure, the crude product was obtained by normal phase silica gel column purification, and the pure product was further purified by HPLC.
[0221] 1 H NMR (400MHz, MeOD-d 4 ,ppm)δ8.67(s,1H),8.35(d,J=8.5Hz,1H),8.14(s,1H),7.91(d,J=8.3Hz,1H),7.84(d,J=7.1 Hz,1H),7.72-7.68(m,2H),7.29(d,J=8.3Hz,1H).LRMS(ESI)calcd.For C 17 h 13 N 5 O[M+H] + :304.33; found: 304.54.
Embodiment 2
[0222] The preparation of compound shown in embodiment 2, formula 2
[0223]
[0224] The preparation method is the same as in Example 1, and the raw materials used are 4-amino-8-bromoquinoline-3-carboxamide and 5-methyl-1H-indazole-6-boronic acid.
[0225] 1 H NMR (400MHz, MeOD-d 4 ,ppm)δ8.60(s,1H),8.36(d,J=7.3Hz,1H),8.05(s,1H),7.73-7.67(m,3H),7.43(s,1H),2.02(s ,3H).LRMS(ESI)calcd.For C 18 h 15 N 5 O[M+H] + :318.36; found: 318.70.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com