Multi-active base particle dyeing process
A process and modified technology, applied in the field of cotton fabric dyeing, can solve the problems of low effective utilization rate, poor stability, large dosage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
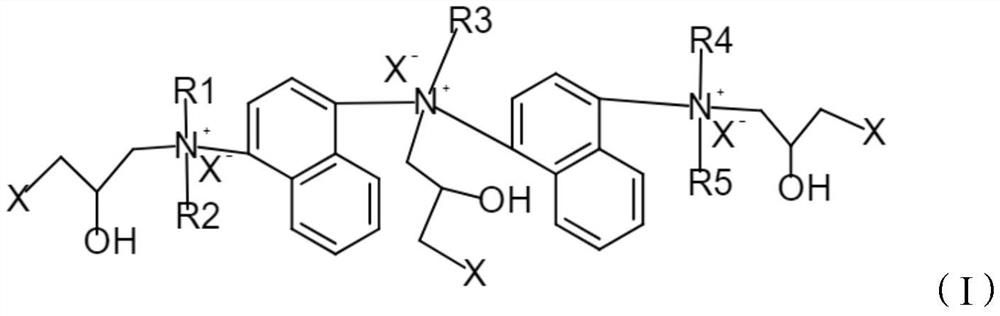
[0048] 1. Preparation of cationic modifier 1 containing the following formula
[0049]
[0050] (1) Add 1,4 dichloronaphthalene in the reaction vessel, pass into ammonia gas at a molar ratio of ammonia: 1,4 dichloronaphthalene about 3:2, react at 200°C under the action of Fe catalyst for about 4 Hours, an oily crude product was obtained, and 85% ethanol solution was slowly added to the crude product for recrystallization and purification; the product was dissolved in anhydrous ether;
[0051] (2) Add methyl iodide CH to the ether solution of the purified product obtained in (1) at a molar ratio of about 1:10 3 1, react at room temperature for about 5 hours to obtain an oily crude product; slowly add 70% ethanol solution to the product for recrystallization and purification; dissolve the product in anhydrous ether;
[0052] (3) Add the ether solution of the purified product obtained in (2) into a synthesis kettle equipped with a reflux condenser, start the agitator, add 10 ...
Embodiment 2
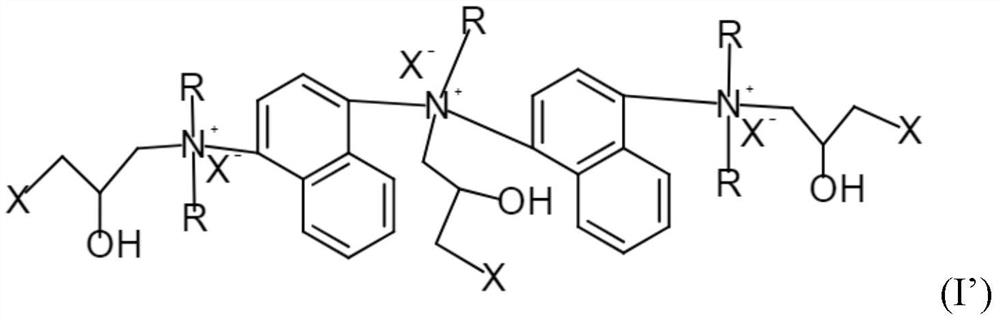
[0062] 1. Preparation of cationic modifier 2 containing the following formula
[0063]
[0064] (1) Add 1,4 dichloronaphthalene in the reaction vessel, pass into ammonia gas at a molar ratio of ammonia: 1,4 dichloronaphthalene about 3:2, react at 200°C under the action of Fe catalyst for about 4 Hours, an oily crude product was obtained, and 85% ethanol solution was slowly added to the crude product for recrystallization and purification; the product was dissolved in anhydrous ether;
[0065] (2) Add iodoethane ICH to the ether solution of the purified product obtained in (1) at a molar ratio of about 1:10 2 CH 3 , react at room temperature for about 5 hours to obtain an oily crude product; slowly add 70% ethanol solution to the product for recrystallization and purification; dissolve the product in anhydrous ether;
[0066] (3) Add the ether solution of the purified product obtained in (2) into a synthesis kettle equipped with a reflux condenser, start the agitator, add ...
Embodiment 3
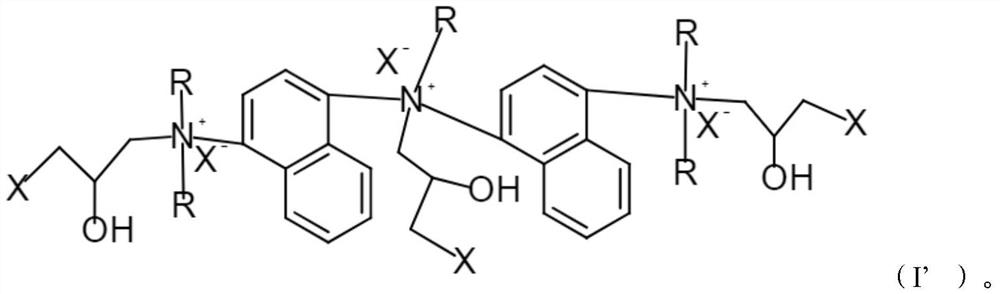
[0076] 1. Preparation of cationic modifier 3 containing the following formula
[0077]
[0078] (1) Add 1,4 dichloronaphthalene in the reaction vessel, pass into ammonia gas at a molar ratio of ammonia: 1,4 dichloronaphthalene about 3:2, react at 200°C under the action of Fe catalyst for about 4 Hours, an oily crude product was obtained, and 85% ethanol solution was slowly added to the crude product for recrystallization and purification; the product was dissolved in anhydrous ether;
[0079] (2) Add iodoisopropane ICH (CH 3 ) 2 , react at room temperature for about 12 hours to obtain an oily crude product; slowly add 70% ethanol solution to the product for recrystallization and purification; dissolve the product in anhydrous ether;
[0080] (3) Add the ether solution of the purified product obtained in (2) into a synthesis kettle equipped with a reflux condenser, start the agitator, add 10 times the molar amount of HCl solution dropwise to the synthesis kettle, and then ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com