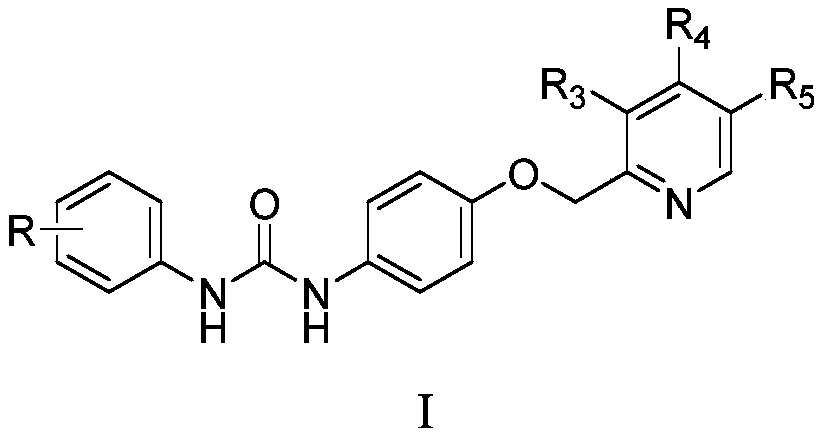
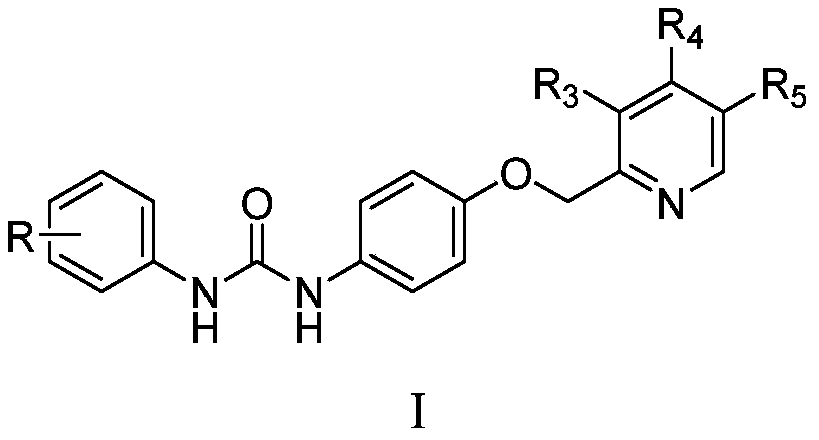
1-aryl-3-[4-(pyridine-2-ylmethoxy) phenyl urea compound and application
A technology of -3-{4-{, compounds, applied in the field of medicine, achieving good development and application prospects and novel structure types
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
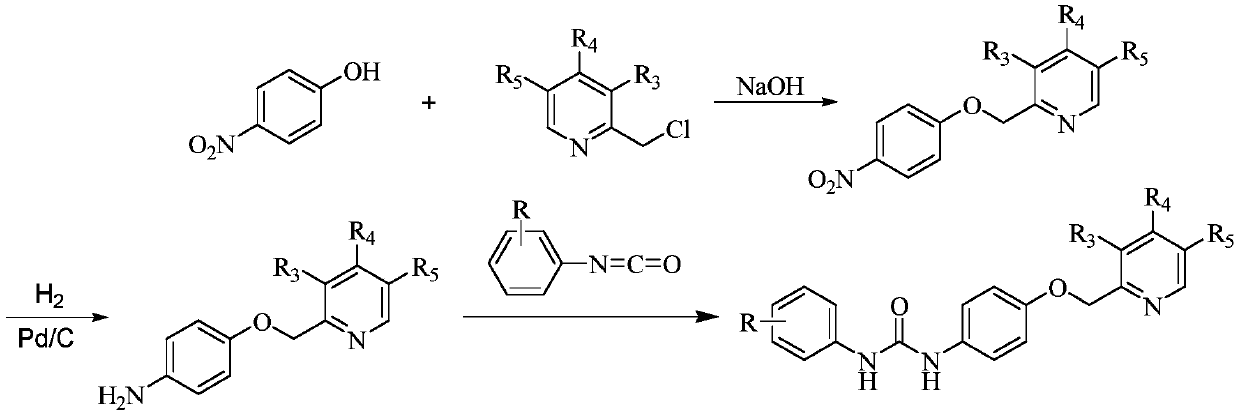
[0054] The following schemes outline the preparative steps for preparing compounds of the invention.
[0055]
[0056] Among them, R, R 3 , R 4 , R 5 as mentioned earlier.
[0057] The present invention is described in detail with the following examples. However, it should be understood that the present invention is not limited to the specific recited examples below.
Embodiment 1
[0058] Example 1: 1-(3,5-difluorophenyl)-3-{4-{[4-(3-methoxypropoxy)-3-methylpyridin-2-yl]methoxy }phenyl}urea (compound Z01)
[0059] Step A: Preparation of 4-(3-methoxypropoxy)-3-methyl-2-[(4-nitrophenoxy)methyl]pyridine
[0060] Add 4-nitrophenol (13.91g, 0.10mol), 2-chloromethyl-3-methyl-4-(3-methoxypropoxy)pyridine hydrochloride (26.62g , 0.10mol) and absolute ethanol (200mL). A 3 mol / L sodium hydroxide aqueous solution (100 mL) was added dropwise at room temperature. After the dropwise addition, react at room temperature for 6h. After the reaction, part of the ethanol was distilled off under reduced pressure, and 200 mL of water was added to the residue to precipitate a large amount of white solid. The filter cake was filtered with suction, washed with water, and dried in air to obtain 30.68 g of white solid, with a yield of 92.6%. The product was used directly in the next step without further purification.
[0061] Step B: Preparation of 4-{[4-(3-methoxypropoxy)-3-m...
Embodiment 2
[0065] Example 2: 1-{4-{[4-(3-methoxypropoxy)-3-methylpyridin-2-yl]methoxy}phenyl}-3-[3-(trifluoro Preparation of methyl) phenyl] urea (compound Z02)
[0066] Referring to the preparation method of Example 1, 0.16 g of white solid was obtained with a yield of 41%, m.p.: 126.2-127.3°C; 1 H NMR (400MHz, DMSO-d 6 )δ8.98(s,1H),8.61(s,1H),8.27(d,J=5.7Hz,1H),8.01(s,1H),7.56(d,J=8.5Hz,1H),7.51- 7.48(m,1H),7.36(d,J=2.2Hz,1H),7.34(d,J=2.3Hz,1H),7.29(d,J=7.5Hz,1H),7.00(d,J=5.8 Hz,1H),6.98(d,J=2.2Hz,1H),6.96(d,J=2.2Hz,1H),5.11(s,2H),4.11(t,J=6.2Hz,2H),3.49( t,J=6.2Hz,2H),3.25(s,3H),2.18(s,3H),2.02-1.96(m,3H).ESI-MS(m / z):490.3([M+H] + ); 512.1 ([M+Na] + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com