Low-cost preparation method for cefixime
A technology of cefixime and cefixime active ester, which is applied in the field of preparation of cefixime, can solve the problems of high process environmental protection pressure, etc., and achieve the effect of short process route, low cost and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
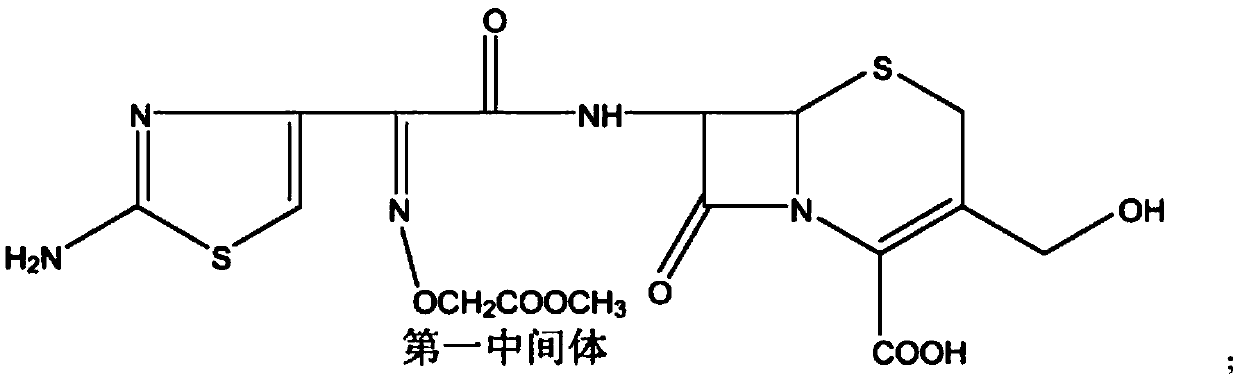
[0026] The present invention provides a low-cost preparation method of cefixime, including:
[0027] S1. React with D-7-ACA (hydroxymethyl-7-aminocephalosporanic acid, CAS number: 15690-38-7) and cefixime active ester (MICA active ester, CAS number: 246035-38-1) The first intermediate is obtained; specifically, the mass ratio of D-7-ACA to cefixime active ester is 1:1.7 to 3.5, that is, 1:1.7 to 1:3.5, and the mass ratio of D-7-ACA and Add tetrahydrofuran and water to the active ester of cefixime, and slowly add tetrahydrofuran and triethylamine dropwise while stirring at -10~30℃, keep at -10~30℃, and detect by HPLC until D-7-ACA The residue on the HPLC chart is less than 0.5% (area normalization method) to obtain the reaction liquid of the first intermediate, the reaction liquid of the first intermediate is extracted with an organic solvent, the organic solvent phase after extraction is recovered, and the aqueous phase Adjust the pH to 2.5-4.5 with an acid solution, and filter ...
Embodiment 1
[0038] In a 1000 ml reaction flask, add 40.0 g of D-7-ACA, 80 g of cefixime active ester, 280 ml of THF (tetrahydrofuran), and 268 ml of pure water. While stirring, control the temperature to 0-5°C, slowly add 40ml THF+28ml TEA (triethylamine) dropwise, maintain the temperature for about 1 hour and complete the addition. After the addition, maintain the temperature for 10 hours, and HPLC detects that the residual D-7-ACA is low. The reaction is complete at 0.5%. It was extracted twice with 400ml ethyl acetate, and the ethyl acetate phase was recovered after extraction. The aqueous phase was adjusted to pH 3.9-4.0 with 9% dilute hydrochloric acid to crystallize, filtered and dried to obtain about 80.2g of the first intermediate.
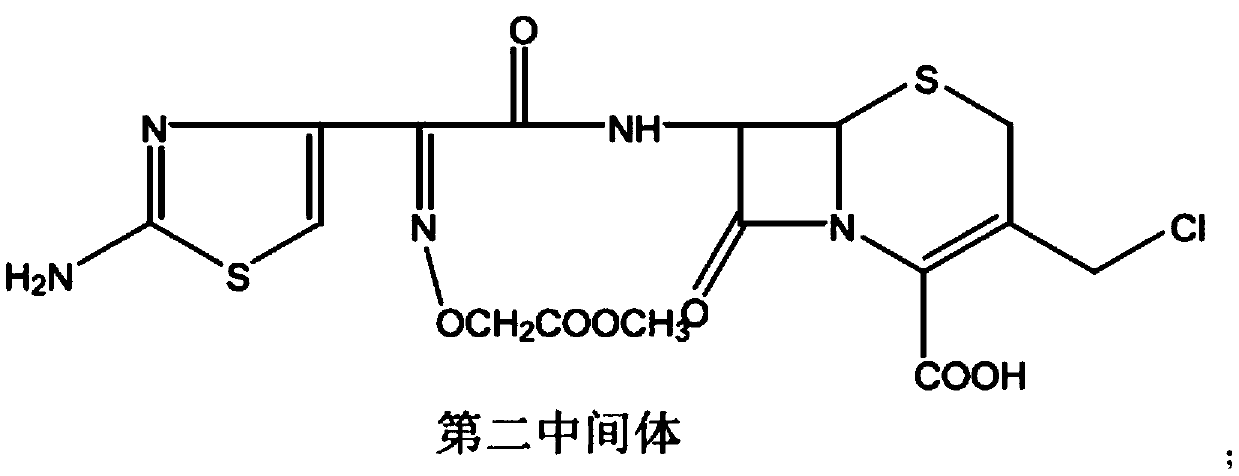
[0039] In a dry and anhydrous reaction flask, add 60.0g of the first intermediate, add 250ml of dichloromethane, add 20ml of DMA, stir and reduce the temperature to 0~10℃, add 32g of phosphorus pentachloride in batches, keep the temperature and stir for ...
Embodiment 2
[0042] In a 1000 ml reaction flask, add 40.0 g of D-7-ACA, 72 g of cefixime active ester, 280 ml of THF (tetrahydrofuran), and 268 ml of pure water. While stirring, control the temperature at -10~5℃, slowly add 40ml THF+28ml TEA (triethylamine) dropwise, maintain the temperature for about 1 hour and complete the addition. After the addition, maintain the temperature and react for 12 hours. HPLC detects D-7-ACA residue Less than 0.5% of the reaction is complete. It was extracted twice with 400ml ethyl acetate, and the ethyl acetate phase was recovered after extraction. The aqueous phase was adjusted to pH 3.3-3.5 with 9% dilute hydrochloric acid to crystallize, filtered and dried to obtain about 79.3g of the first intermediate.
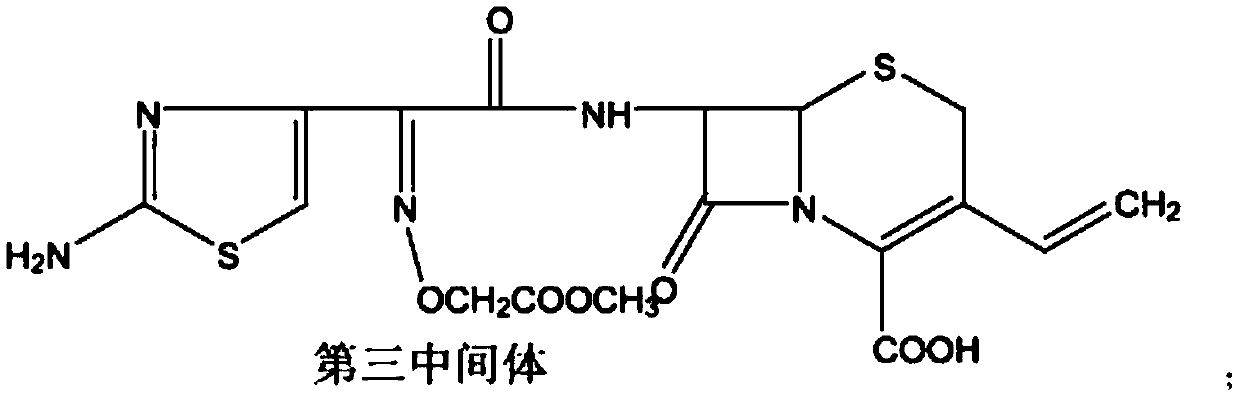
[0043] In a dry and anhydrous reaction flask, add 60.0g of the first intermediate, add 250ml of dichloromethane, add 20ml of DMA, stir and reduce the temperature to -10~0℃, add 30g of phosphorus pentachloride in batches, maintain the temperature and stir ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com