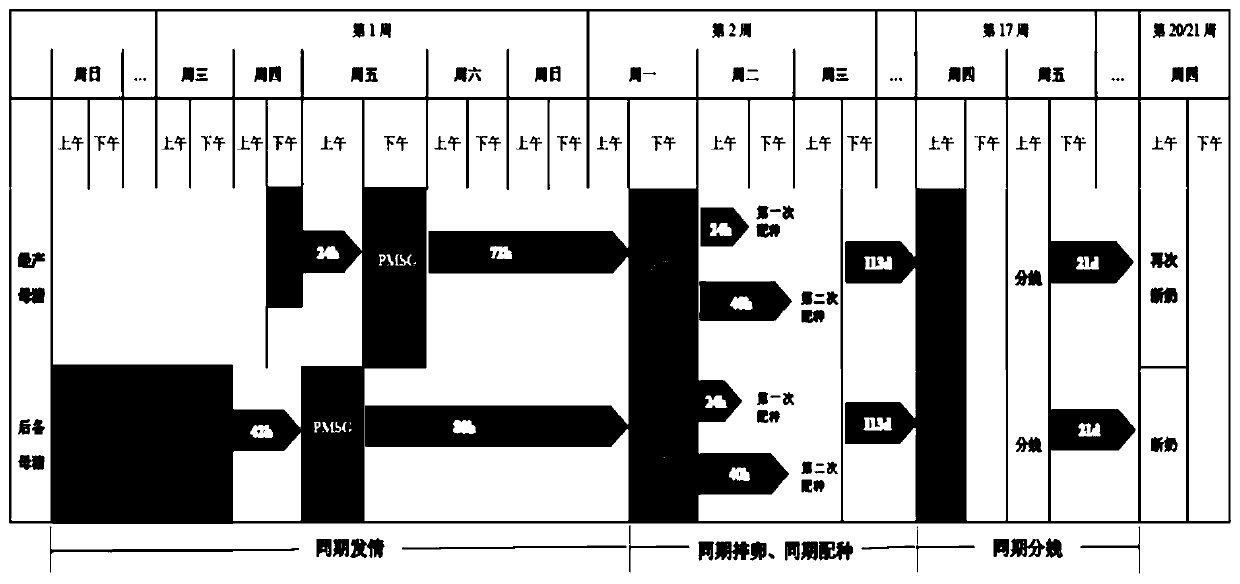
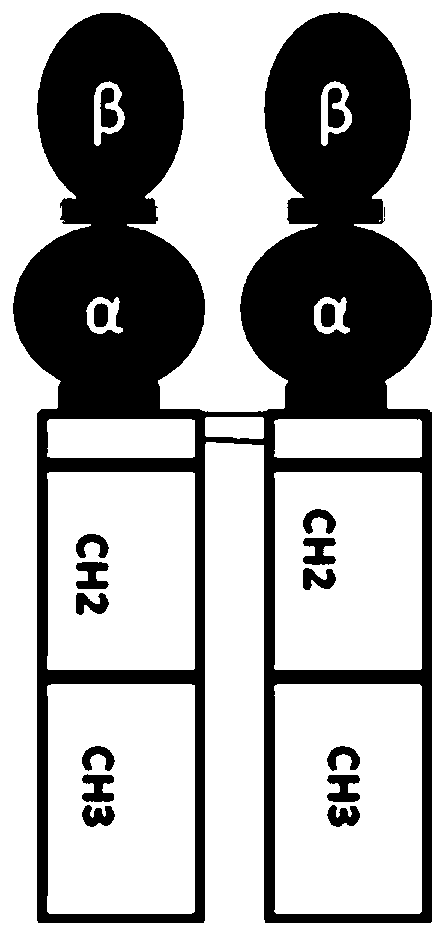
Application of long-acting recombinant FSH (follicle-stimulating hormone) fusion protein to batch production of sows
A fusion protein and sow technology, which is applied to medical preparations containing active ingredients, fusion polypeptides, drug combinations, etc., can solve the problems of no long-acting recombinant FSH fusion protein, low expression of recombinant hFSH, frequent injections, etc. , to achieve the effects of improving reproductive efficiency and breeding level, shortening average litter size, and increasing estrus rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Embodiment 1 Preparation of recombinant FSH-Fc fusion protein
[0073] 1.1 Construction of gene expression vector encoding recombinant hFSH-Fc fusion protein
[0074] The gene sequence design was optimized based on the preferred codons of CHO cells, and the optimized fusion gene encoding the signal peptide and hFSH protein β-chain mature peptide, CTP and hFSH protein α-chain mature peptide was synthesized by artificial synthesis. The synthesized DNA fragment is inserted between the EcoRV restriction sites in the transfer vector such as pUC57 to obtain the hFSH plasmid (ie phFSH), and the correctness of the inserted sequence is verified by DNA sequencing method.
[0075] The expression vector was transformed according to pcDNA3.0. First remove the original NeoR / KanR (neomycin / kanamycin) of pcDNA3.0 and replace it with the anti-apoptotic gene BIRC2. BIRC2 gene binds to activated Caspase-3 / 7 and relies on E3 ubiquitin ligase Activity, mediate Caspase-3 / 7 degradation, the...
Embodiment 2
[0090] Preparation of embodiment 2 recombinant FSH-CTP fusion protein
[0091] 2.1 Construction of gene expression vector encoding recombinant hFSH-CTP fusion protein
[0092] The gene sequence design is optimized based on the preferred codons of CHO cells, and the optimized DNA fragment encoding hFSH protein β chain mature peptide, CTP, bGH termination signal, CMV promoter and hFSH α chain mature peptide is synthesized by artificial synthesis , with SpeI and EcoRI restriction sites at both ends. The synthesized DNA fragment was inserted between the EcoRV restriction sites in a transfer vector such as pUC57 to obtain a hFSH-CTP plasmid (ie, phFSH-CTP), and the correctness of the inserted sequence was verified by DNA sequencing.
[0093] The expression vector was transformed according to pcDNA3.0. First remove the original NeoR / KanR (neomycin / kanamycin) of pcDNA3.0 and replace it with the anti-apoptotic gene BIRC2. BIRC2 gene binds to activated Caspase-3 / 7 and relies on E3 ub...
Embodiment 3
[0110] The pharmacokinetic determination of embodiment 3 recombinant FSH-Fc fusion protein
[0111] The test is divided into the recombinant hFSH-vIgG2-hFc, hFSH-vIgG1-hFc, pFSH-vIgG2-hFc, pFSH-vIgG1-hFc, pFSH-pFc fusion protein administration group provided by the present invention and porcine pituitary FSH (commercial product) administration group. For the medicine group, 5 male SD rats with a body weight of 220 ± 10 g were selected for each group, and were injected subcutaneously at a dose of 30 IU / kg respectively.
[0112] Pig pituitary FSH group took blood at 1h, 2h, 4h, 6h, 8h, 12h, 24h, 36h, 60h after administration, recombinant hFSH-vIgG2-hFc, hFSH-vIgG1-hFc, pFSH-vIgG2-hFc, pFSH-vIgG1 -hFc, pFSH-pFc fusion protein were collected at 0h, 2h, 6h, 12h, 24h, 32h, 48h, 56h, 72h, 80h, 96h, 104h, 120h after administration, and centrifuged at 4°C and 3000rpm After 5 minutes, the serum was aspirated and stored at -20°C. ELISA kit (BIOCHECK, USA) was used to measure FSH immune...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com