High strength and high toughness self-repairing thermoplastic polyurethane urea elastomer and preparation method thereof
A polyurethane urea and high-toughness technology is applied in the field of high-strength and high-toughness self-healing thermoplastic polyurethane urea elastomer and its preparation, achieving high application value, maintaining integrity and functionality, and improving self-healing ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
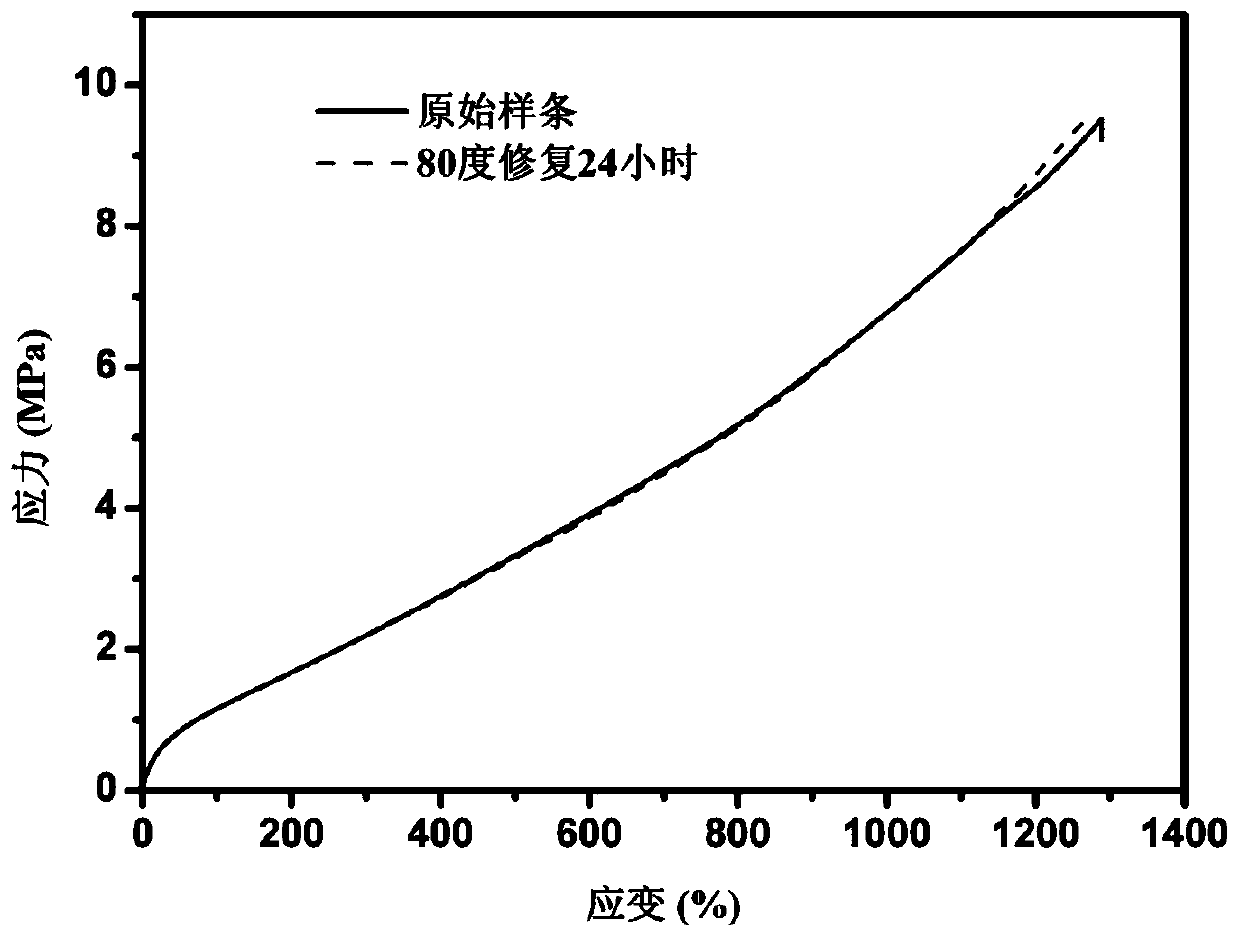
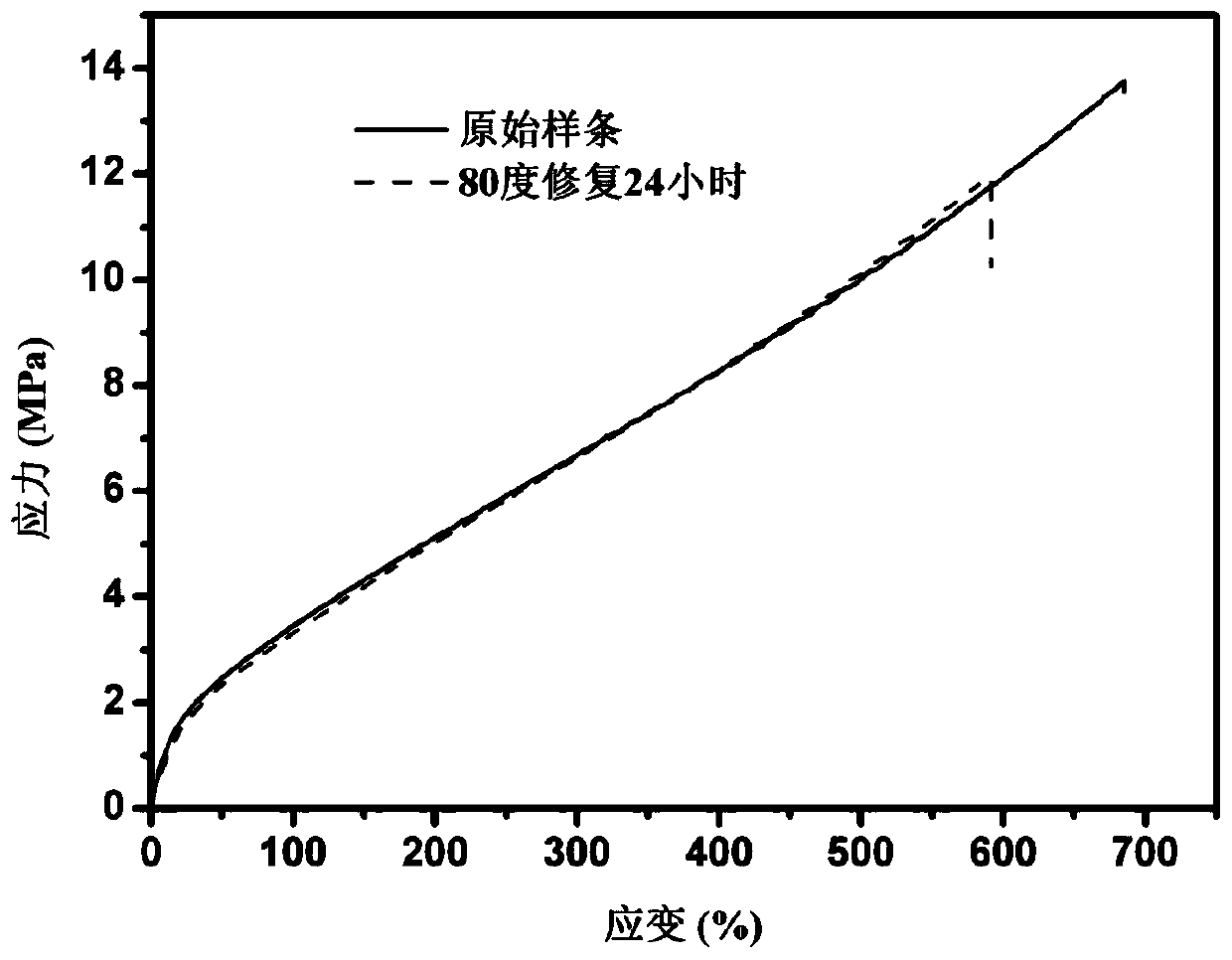
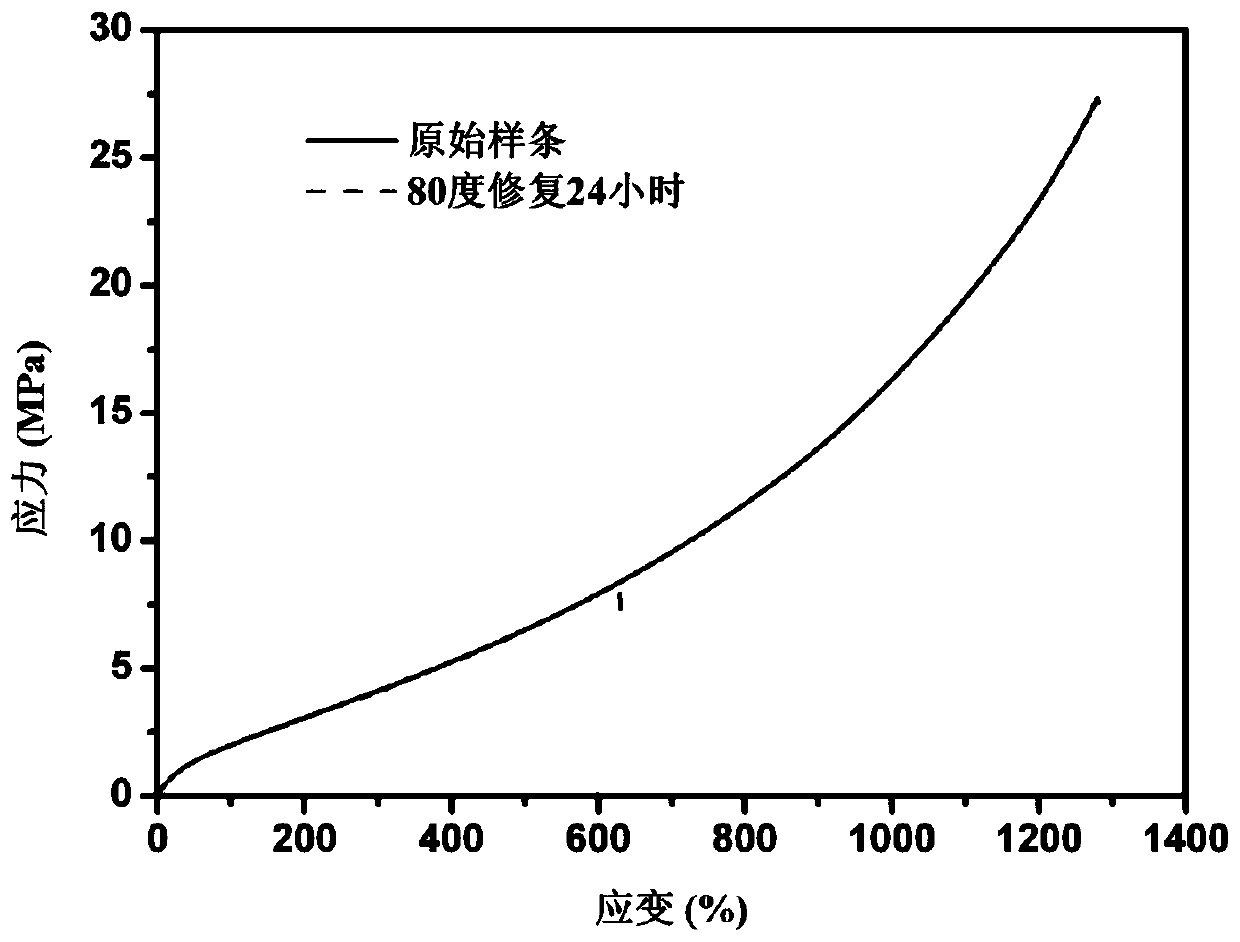
Image
Examples
Embodiment 1
[0049] Step (1): Set the R value to 2.0, put 0.010mol (20.000g) PPG2000 into the reactor, raise the temperature to 120°C, keep the vacuum at -0.095MPa and remove water for 1 hour while stirring, then lower the temperature to 80°C , pass into nitrogen for protection, put in 0.020mol (5.005g) MDI-100, stir and react for 1.5 hours, add 2.000×10 -6 mol of dibutyltin dilaurate continued to react for 1.5 hours to obtain an isocyanate group-terminated polyurethane prepolymer, which was protected under nitrogen for later use.
[0050] Step (2): Set the x / y ratio to 1.5, dissolve 0.002mol (0.218g) 2,6-diaminopyridine in 10mL N,N-dimethylformamide, and put it into the prepolymer obtained in step (1) In the process, the temperature was maintained at 80° C., protected by nitrogen gas, and after 2 hours of reaction, 0.016 mol (2.057 g) of p-chlorophenol was added to continue the reaction for 1 hour to obtain a polyurethane urea prepolymer blocked by an end-capping agent.
[0051] Step (3)...
Embodiment 2
[0053] Step (1): Set the R value to 2.0, put 0.010mol (20.000g) PPG2000 into the reactor, raise the temperature to 120°C, keep the vacuum at -0.095MPa and remove water for 1 hour while stirring, then lower the temperature to 80°C , pass into nitrogen for protection, put in 0.020mol (5.005g) MDI-100, stir and react for 1.5 hours, add 2.000×10 -6 mol of dibutyltin dilaurate continued to react for 1.5 hours to obtain an isocyanate group-terminated polyurethane prepolymer, which was protected under nitrogen for later use.
[0054] Step (2): Set the x / y ratio to 1, set 2.500×10 -3 mol (0.273g) of 2,6-diaminopyridine was dissolved in 10mL N,N-dimethylformamide, and put into the prepolymer obtained in step (1), the temperature was kept at 80°C, and the nitrogen gas was used for protection. After 2 hours of reaction , put in 0.015mol (1.928g) of p-chlorophenol and continue the reaction for 1 hour to obtain a polyurethane urea prepolymer capped by a capping agent.
[0055] Step (3): ...
Embodiment 3
[0057] Step (1): Set the R value to 2.0, put 0.010mol (20.000g) PPG2000 into the reactor, raise the temperature to 120°C, keep the vacuum at -0.095MPa and remove water for 1 hour while stirring, then lower the temperature to 80°C , pass into nitrogen for protection, put in 0.020mol (5.005g) MDI-100, stir and react for 1.5 hours, add 2.000×10 -6 mol of dibutyltin dilaurate continued to react for 1.5 hours to obtain an isocyanate group-terminated polyurethane prepolymer, which was protected under nitrogen for later use.
[0058] Step (2): Set the x / y ratio to 0.5, and set 3.333×10 -3 mol (0.363g) of 2,6-diaminopyridine was dissolved in 10mL of N,N-dimethylformamide, and put into the prepolymer obtained in step (1), the temperature was kept at 80°C, protected by nitrogen gas, and reacted for 2 hours After that, input 1.333×10 -2 mol (1.714g) of p-chlorophenol continued to react for 1 hour to obtain a polyurethane urea prepolymer terminated by an end-capping agent.
[0059] Ste...
PUM
| Property | Measurement | Unit |
|---|---|---|
| elongation at break | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com