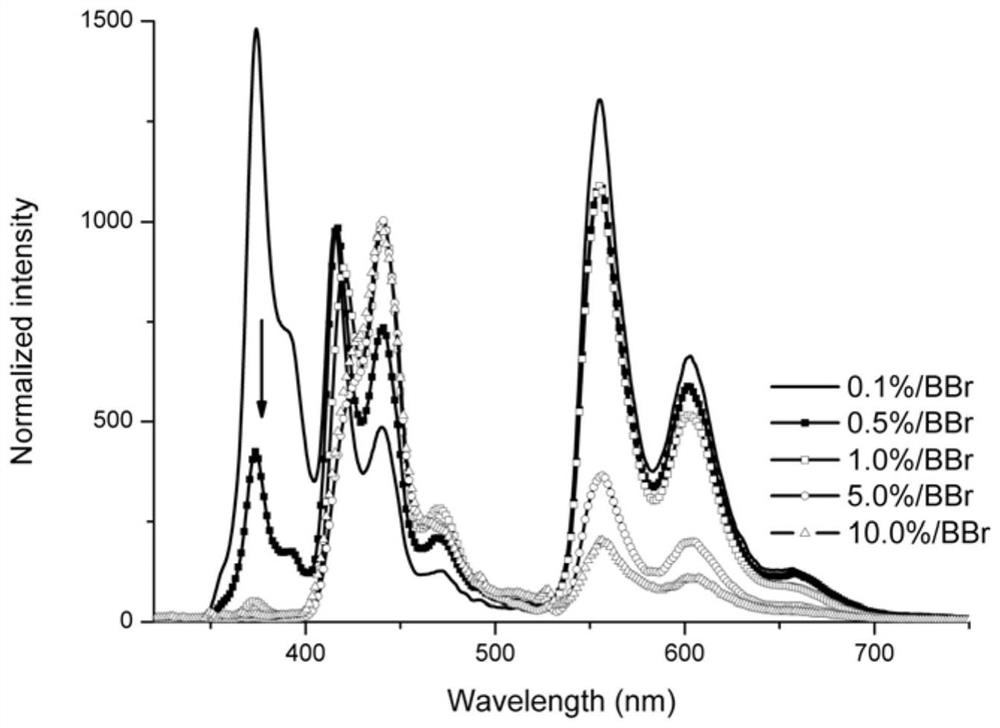
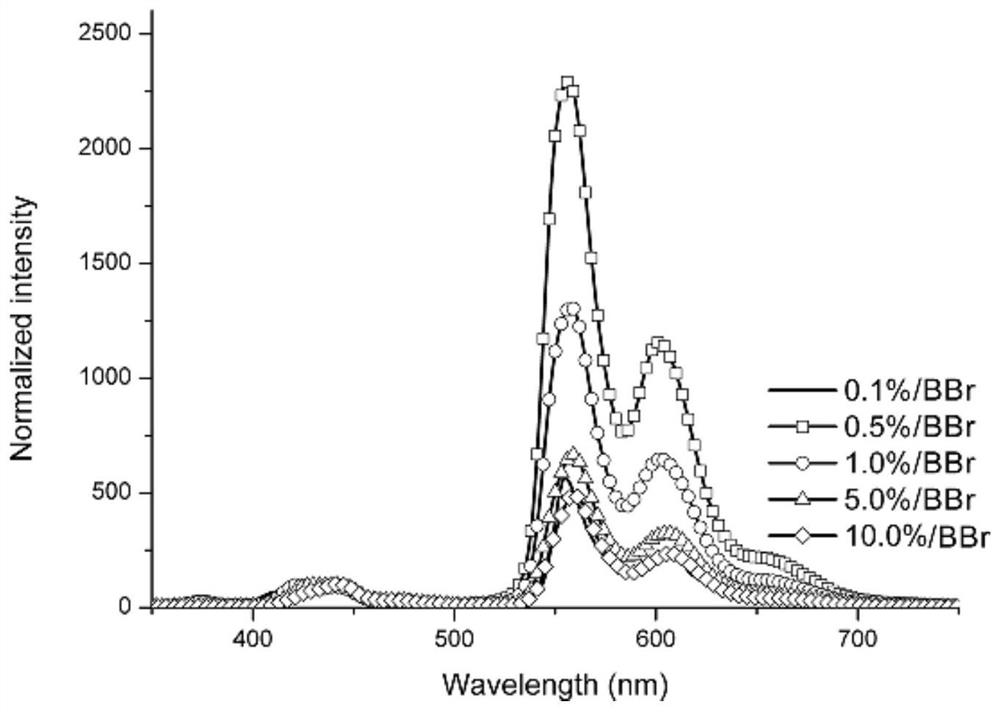
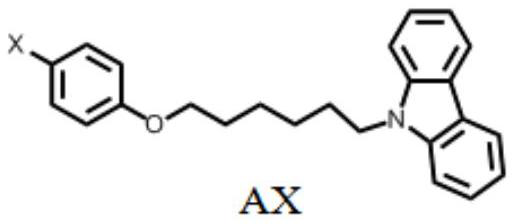
A pure organic material with strong yellow afterglow and its preparation method and application
A technology of organic materials and luminescent materials, applied in the field of organic afterglow luminescent materials, can solve problems such as low luminous efficiency, and achieve the effects of simple structure, excellent afterglow luminescent performance, and high phosphorescence quantum efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of BBr: benzo[b]carbazole (0.1 g, 0.46 mmol) was dissolved in 10 mL of DMF, and stirred for 10 minutes under an ice-water bath. NaH (0.06 g, 60%) was slowly added to the above solution, and stirring was continued at 0° C. for 1 hour. Add 1-bromo-4-(5-bromophenoxy)benzene (0.17g dissolved in 5mL DMF) solution dropwise to the above solution, continue stirring for 2 hours, pour into 200mL water, and filter with suction to obtain a light yellow solid. Column chromatography purification (petroleum ether / CH 2 Cl 2 = 2 / 1). Yield: 17%. Elemental analysis: theoretical value: C, 71.19; H, 5.55; N, 2.96; actual value: C, 71.15; H, 5.58; N, 2.93. 1 H NMR (400MHz, d6-DMSO) δ8.73(s, 1H), 8.30(d, J=7.6Hz, 1H), 8.08(d, J=8.3Hz, 1H), 8.02(d, J=8.5Hz ,1H),7.97(s,1H),7.60(d,J=8.2Hz,1H),7.54(t,J=7.5Hz,1H),7.48(t,J=7.0Hz,1H),7.39(t ,J=7.0Hz,1H),7.38(d,J=10.0Hz,2H),7.24(t,J=7.4Hz,1H),6.81(J=9.0Hz,2H),4.45(t,J=6.9 Hz,2H),3.88(t,J=6.4Hz,2H),1.87(m,2H),1.65(m,2H),1.44(m,4H). ...
Embodiment 2
[0034] Preparation of ABr: ABr was prepared according to the conditions for the synthesis of BBr. A colorless solid was obtained, yield: 42%. Elemental analysis: theoretical value: C, 68.25; H, 5.73; N, 3.32; actual value: C, 68.28; H, 5.70; N, 3.34. 1 H NMR (400MHz, CDCl 3 )δ8.13(d, J=7.7Hz, 2H), 7.53–7.46(m, 2H), 7.43(d, J=8.1Hz, 2H), 7.40–7.36(m, 2H), 7.28–7.21(m ,2H),6.74(d,J=9.0Hz,2H),4.35(t,J=7.1Hz,2H),3.89(t,J=6.4Hz,2H),2.04–1.90(m,2H),1.80 –1.67(m,2H),1.53–1.40(m,4H). 13 C NMR (101MHz, CDCl3 )δ158.14,140.44,132.22,125.63,122.86,120.40,118.79,116.29,112.66,108.64,67.97,42.94,29.02,28.95,27.06,25.92.HRMS(m / z):[M+H] + calcd.for C 24 h 25 BrNO, 422.1114; found, 422.1110.
Embodiment 3
[0036] Preparation of AI: Prepare AI according to the conditions for synthesizing BBr. A colorless solid was obtained, yield: 54%. Elemental analysis: theoretical value: C, 61.42; H, 5.15; N, 2.98; actual value: C, 61.46; H, 5.13; N, 2.94. 1 H NMR (400MHz, CDCl 3 )δ8.12(s,2H),7.54(s,2H),7.51–7.46(m,2H),7.43(d,J=8.1Hz,2H),7.28–7.21(m,2H),6.64(d ,J=9.0Hz,2H),4.35(t,J=7.1Hz,2H),3.88(t,J=6.4Hz,2H),1.94(m,2H),1.75(m,2H),1.49(m ,4H). 13 C NMR (101MHz, CDCl 3 )δ158.89,140.42,138.17,125.62,122.85,120.39,118.78,116.90,108.63,82.50,67.82,42.93,28.99,28.94,27.05,25.91.HRMS(m / z):[M+H] + calcd.for C 24 h 25 INO, 470.0981; found, 470.0976.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quantum efficiency | aaaaa | aaaaa |
| quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com