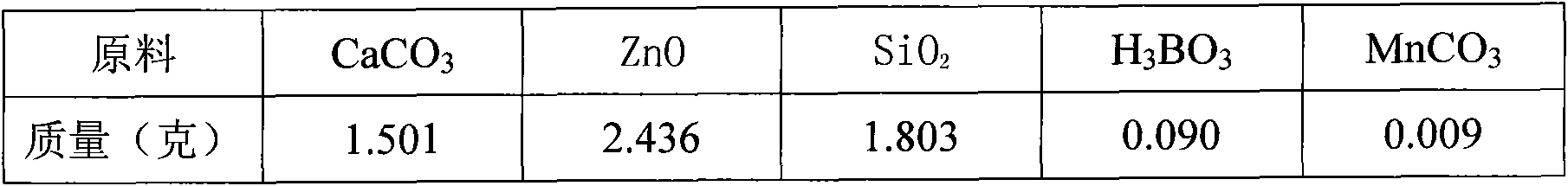
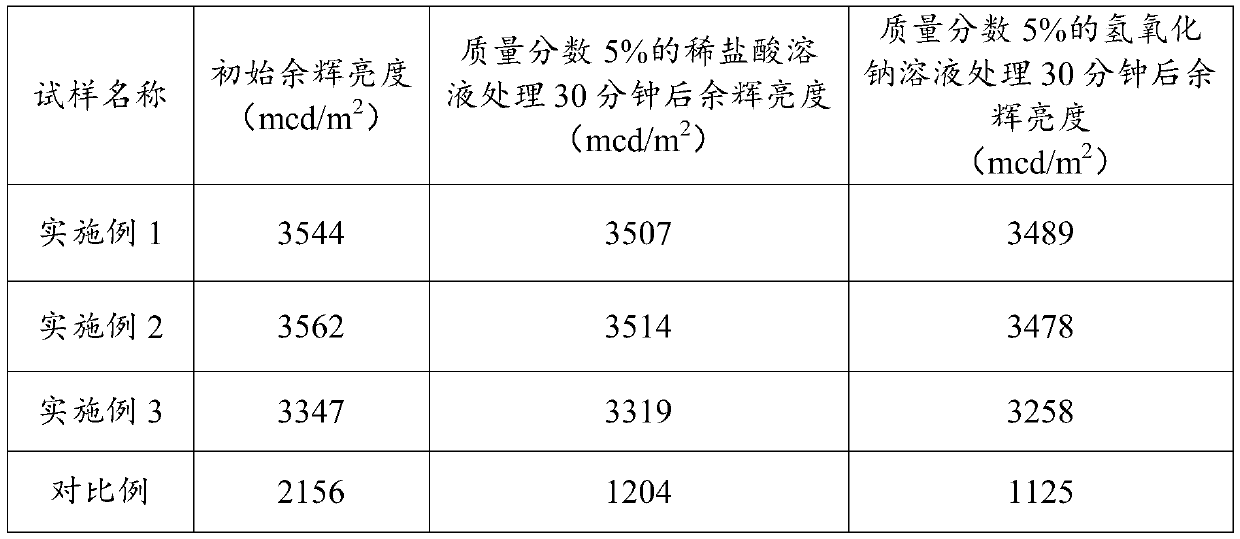
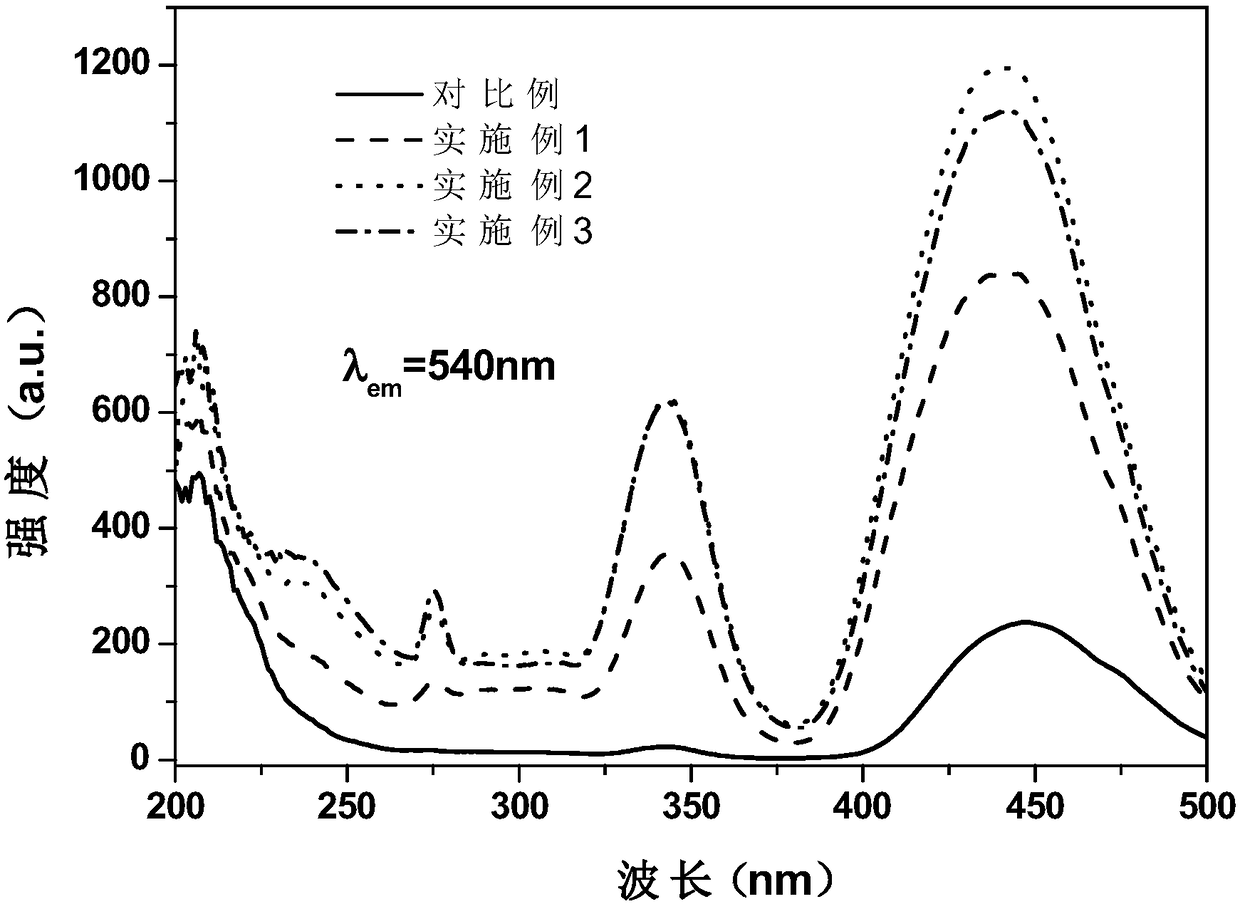
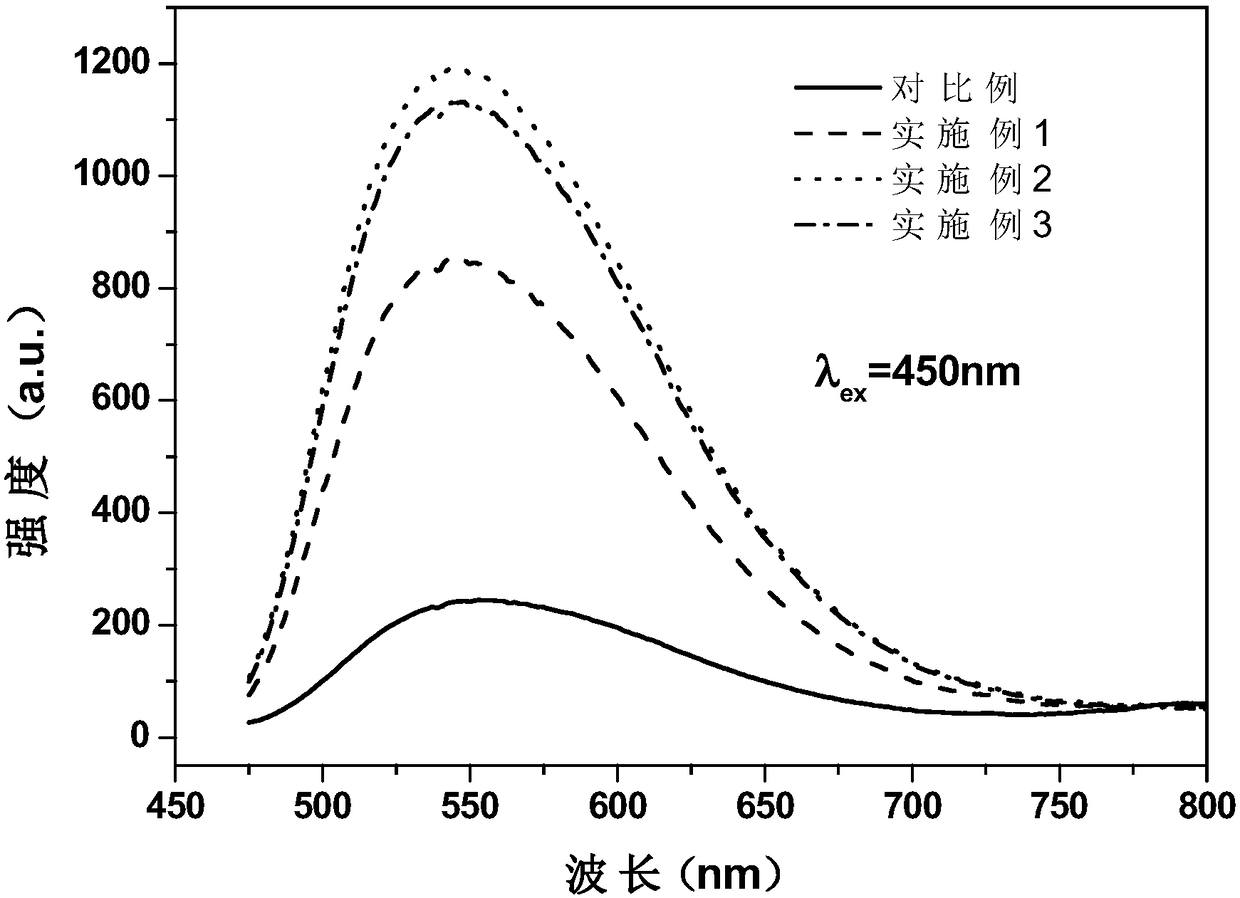
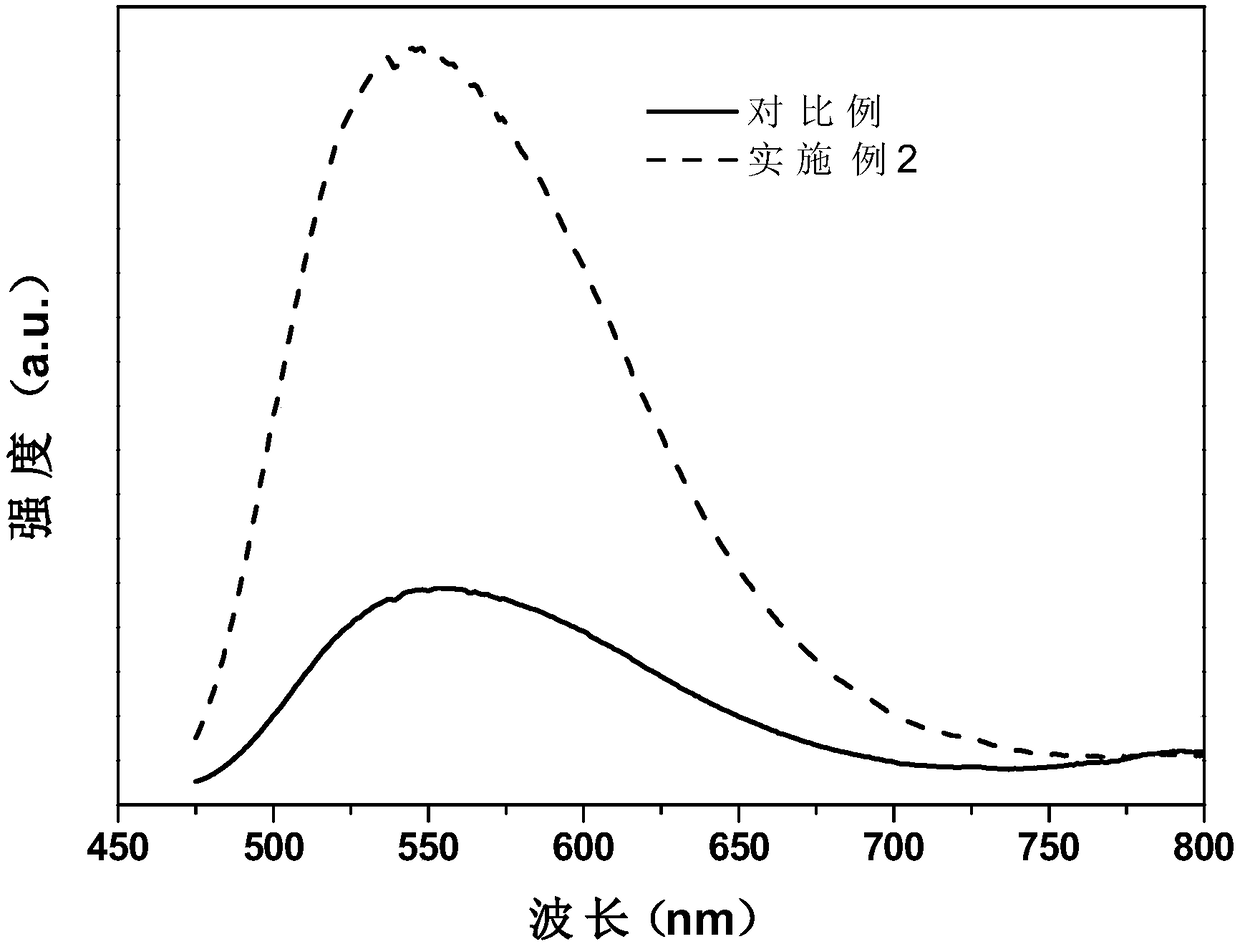
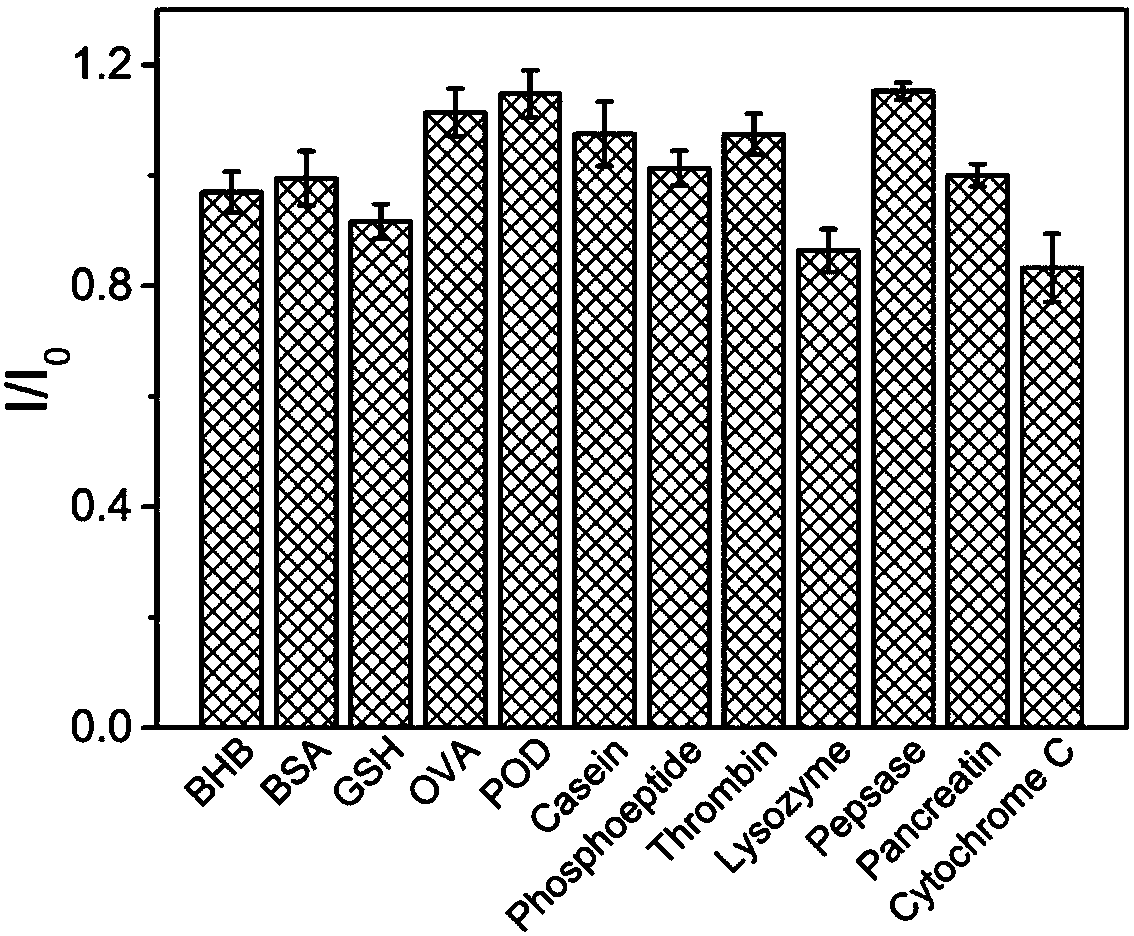
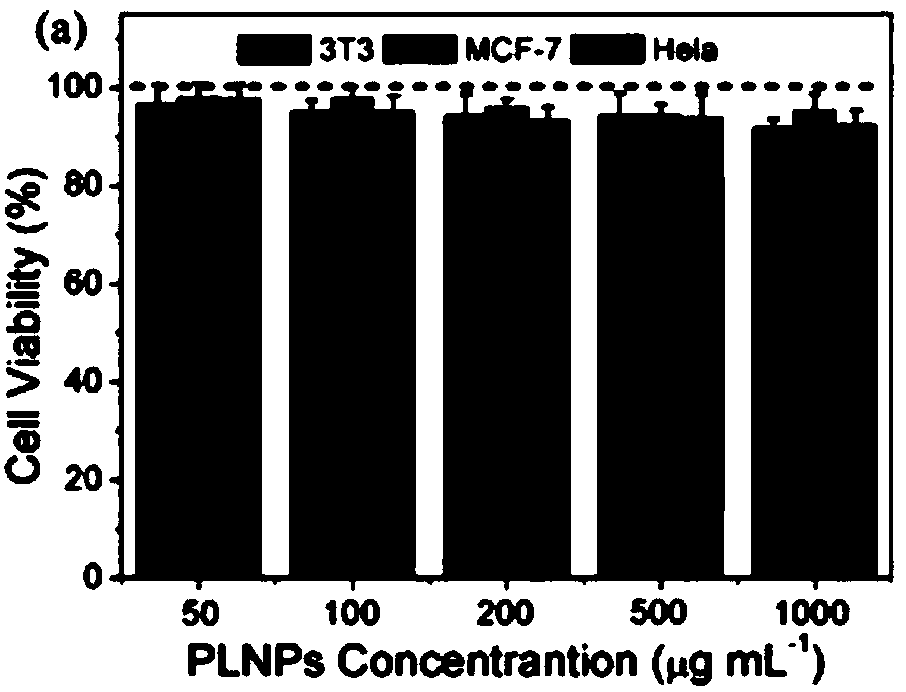
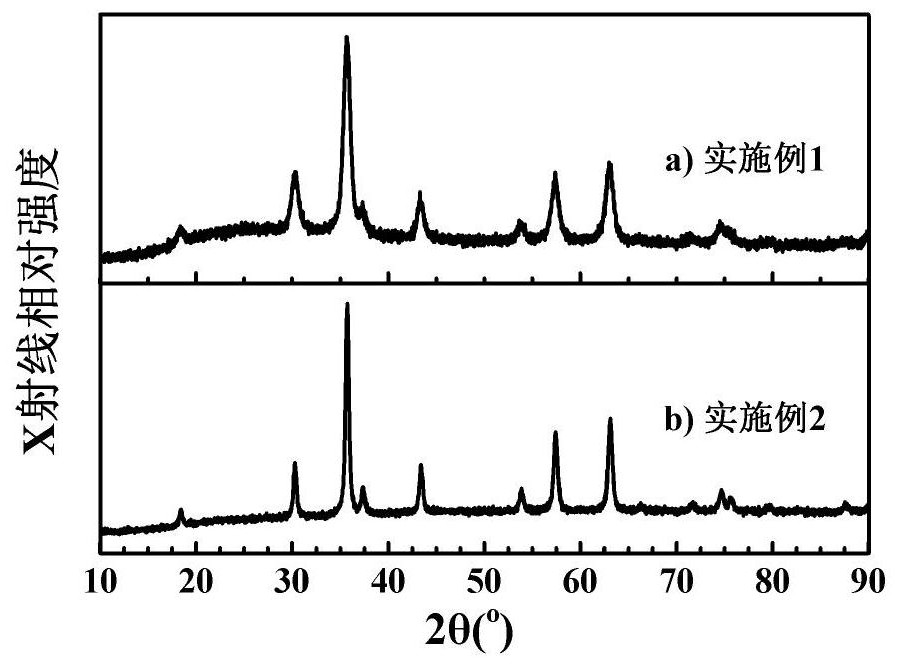
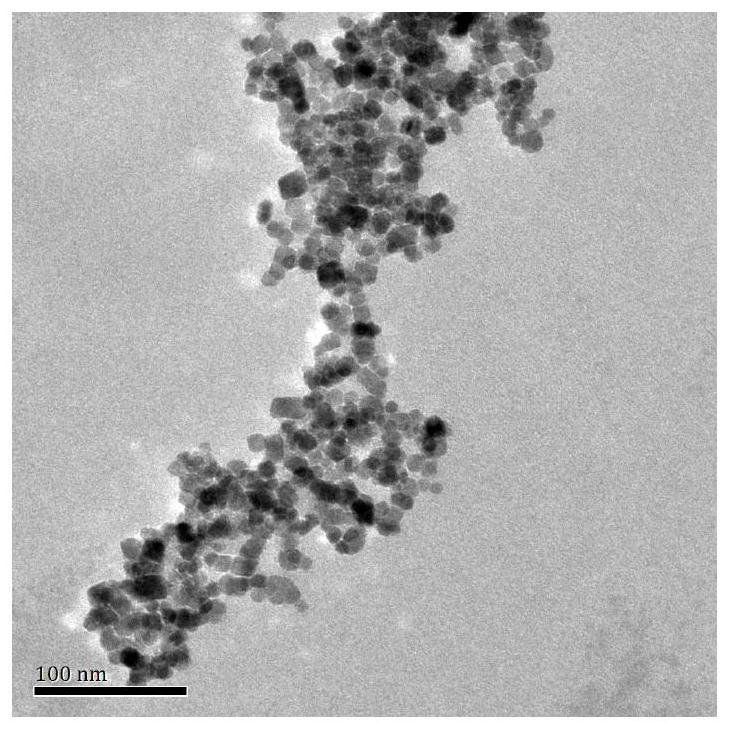
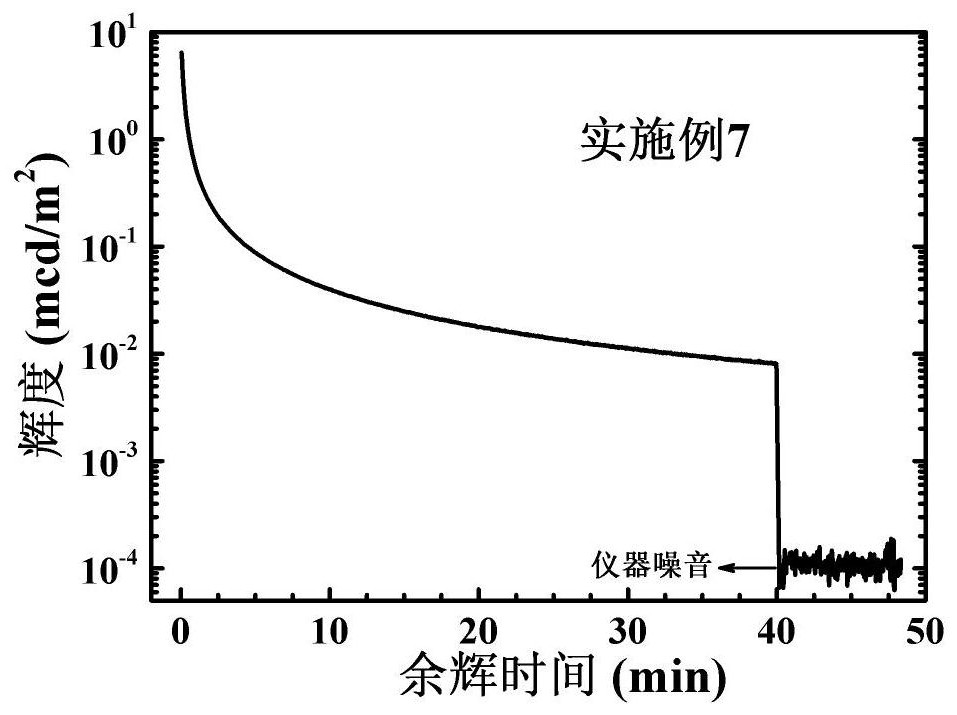
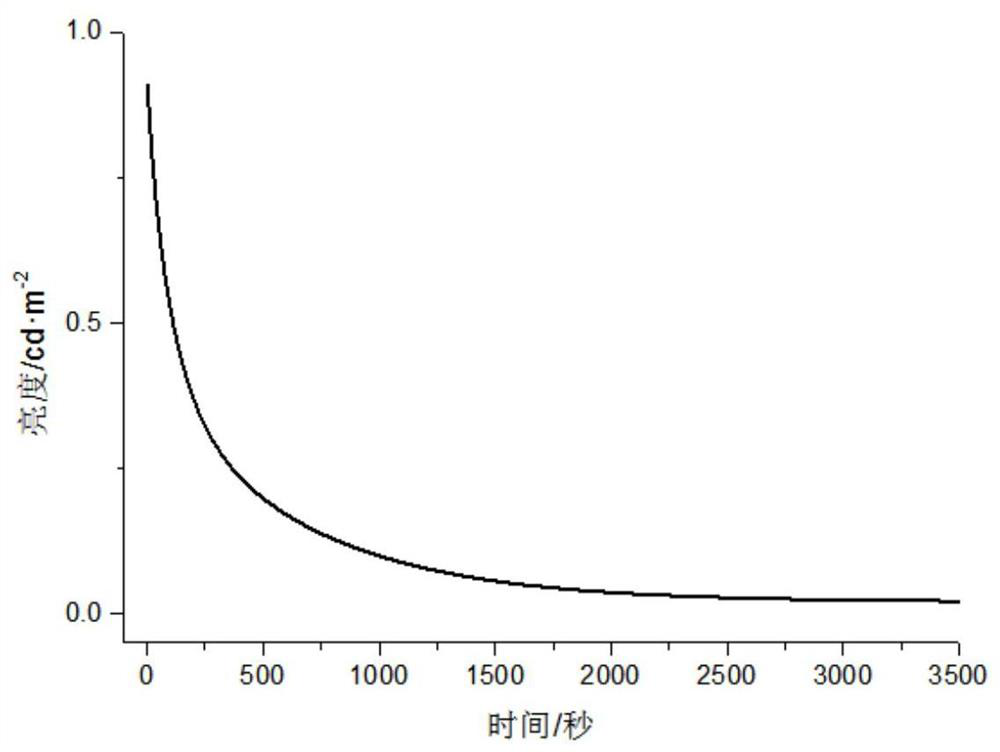
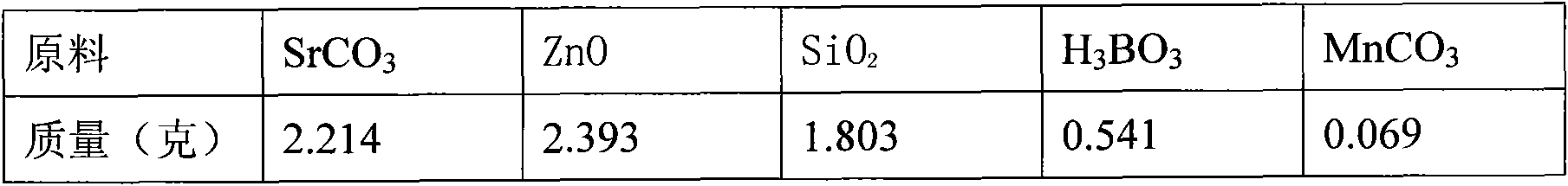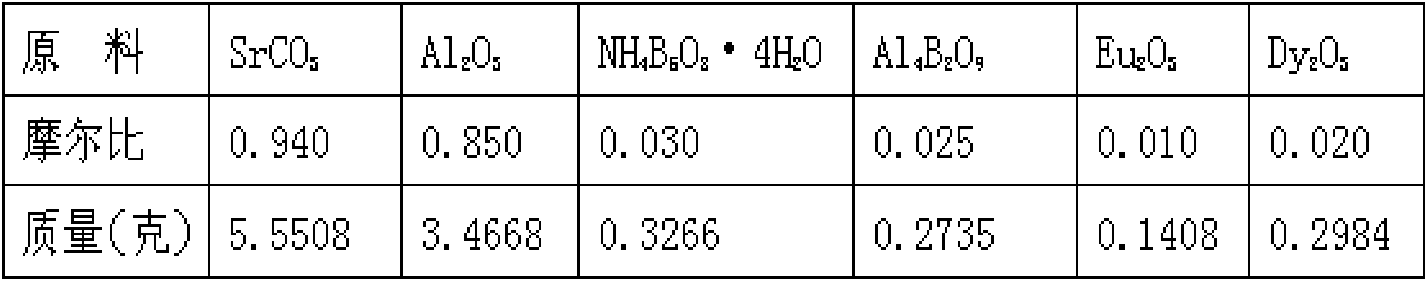Patents
Literature
43results about How to "Excellent afterglow performance" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation of red strontium sulphide long afterglow material
InactiveCN101328405AEasy to controlWell mixedLuminescent compositionsSolubilitySurface-active agents
The invention discloses a method for preparing a red strontium sulphide long afterglow material, comprising a hydrothermal coprecipitation method which comprises the following steps that: (a) water-solubility strontium salt, water-solubility europium salt, water-solubility dysprosium salt, carbamide and water are weight according to the mol ratio of 1: between 0.01 and 0.05: between 0.01 and 0.05: between 4 and 6: between 28 and 32 and are put inside a container to be stirred and dissolved, the mixture is insulated in a sealing state at a temperature of between 80 and 160 DEG C for 5 to 24 hours so that a precursor is obtained; (b) the precursor is subject to filtering and annealing at a temperature of between 900 and 1200 DEG C in the reaction environment for 0. 5 to 2 hours so that the red strontium sulphide long afterglow material is prepared; the water-solubility strontium salt is strontium nitrate or strontium chloride or strontium acetate, the water-solubility europium salt is europium nitrate, europium chloride or polyimide, the water-solubility dysprosium salt is dysprosium nitrate or dysprosium chloride or dysprosium acetate, and a surface active agent is added according to the mol ratio of the water-solubility strontium salt to the surface active agent of 1: between 0.0001 and 0.0003. The method is widely applied to the fields such as building decoration, traffic transportation, military facilities, fire emergency service and goods for everyday consumption.
Owner:HEFEI INSTITUTES OF PHYSICAL SCIENCE - CHINESE ACAD OF SCI
Silicate green long afterglow material and preparation method thereof
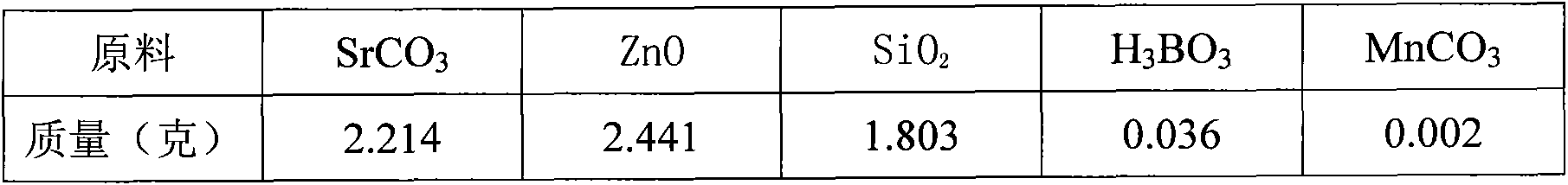
InactiveCN101575510AShine wellExcellent afterglow performanceChemical industryLuminescent compositionsChemical industryReducing atmosphere
The invention relates to a silicate green long afterglow material. The structural formula of a compound of the silicate green long fluorescent lag material is M1-yZn2-xSi2O7:xMn,yRe,zH3BO3, wherein the ratio of (M)O to ZnO to SiO2 is 1:2:2; x, y and z refer to molar coefficient ratio; Mn is an activator; Re is a coactivator; and H3BO3 is an auxiliary solvent. The invention adopts a technical proposal: adopting silicate as a substrate, single Mn ions as the activator, and doped ion Re as the coactivator, and fully mixing the raw materials with the auxiliary solvent in proportion; and igniting the mixture for 2 to 4 hours in a high-temperature furnace at the temperature of between 1,000 and 1,300 DEG C in a reducing atmosphere or in the air, cooling the obtained product along the furnace temperature, and taking out the obtained product. The silicate green long afterglow material overcomes the defect of a few varieties of the prior long afterglow material and particularly overcomes the defects of a few luminous colors, weak after glow brightness, poor water resistance and stability, and the like, and is suitable for passive display and energy-saving illumination in the fields of traffic, building, chemical industry, mine, household electrical appliance and the like.
Owner:XIANGTAN UNIV
Luminescent ceramic with high afterglow intensity and preparation method thereof
ActiveCN110950682AAvoid rain erosion and acid and alkali corrosionExtended service lifeCeramic tilesCorrosion
The invention provides luminescent ceramic with high afterglow intensity. The luminescent ceramic structurally comprises a green body layer, a cover glaze layer, a bonding layer, a luminescent layer,a transparent layer and a pattern layer from bottom to top. According to the luminescent ceramic, the bonding layer is added, so the bonding strength of the luminescent layer and the green body is achieved, the transparent layer is added, so the sealing effect on the luminescent layer can be achieved by the transparent layer, the resistance to rainwater erosion and acid-base corrosion is improved,the service life of luminous ceramic tile can be effectively prolonged, and meanwhile, surface stains and stickers can be cleaned conveniently.
Owner:FOSHAN OCEANO CERAMICS
Rare earth halosilicate red long-afterglow phosphor, and preparation method thereof
InactiveCN102226086AProne to lattice defectsLong afterglowLuminescent compositionsRare earth ionsPhosphor
The invention relates to a type of rare earth halosilicate red long-afterglow phosphor, and a preparation method thereof. Especially, the invention relates to a type of red long-afterglow phosphor prepared from a base material of rare-earth-modified solonetz halosilicate, and a preparation method thereof. The phosphor is characterized in the structural formula of: (1-[alpha])M,2[alpha] / 3Ln)5(SiO4)2X2:[beta]Eu<3+>,[gamma]Ln<,>, wherein M is at least one of Mg and Ca, Ln is at least one of Y, La, and Gd, X is at least one of F and Cl, Ln<,> is at least one of Dy<3+>, Er<3+>, and Bi<3+>, [alpha]=0.1 to 1, [beta]=0.03 to0.07, and [gamma]=0.001 to 0.05. According to the method, solonetz halosilicate is appropriately modified with rare earth, such that rare-earth-modified solonetz halosilicate, which has a structural formula of (M,Ln)5(SiO4)2X2 is obtained. The rare-earth-modified solonetz halosilicate is adopted as a novel base material, and a rare earth ion Eu<3+> is adopted as a luminous ion. According to the luminous ion Eu<3+>, other rare earth ions or non-rare-earth ions are selected as sensitizers, such that the red long-afterglow phosphor with a main peak wavelength of 611nm, an initial luminance of 1200mcd / m<2>, an afterglow period greater than 12 hours, and a good chemical stability is obtained.
Owner:LONGNANXIAN SHUNDE MINGHUI FLUORESCENT MATERIAL
Long-afterglow fluorescent powder applied to LED and preparation method of long-afterglow fluorescent powder
ActiveCN108264898ALong afterglowHigh luminous intensityLuminescent compositionsLuminous intensityTwo step
The invention relates to long-afterglow fluorescent powder applied to an LED and a preparation method of the long-afterglow fluorescent powder. The general chemical formula of the long-afterglow fluorescent powder is (Al-xBx)m-yCy(D1-zEz)8-mO12, wherein A is at least one of Y, Gd, Tb and Lu; B is at least one of La and Yb; C is at least one of Ce, Pr, Nd, Sm, Eu, Dy, Ho, Er, Tm, Ti, Cr and Mn; D is Ga; E is at least one of B, Al, In and Sc; x, y, z and m represent the mole fraction of the corresponding elements; 0< / =x< / =0.2, 0.0001< / =y< / =0.2, 0< / =z< / =0.8, and 2.5< / =m< / =3.5. The preparation method adopts a two-step sintering process which uses oxidization atmosphere first and then uses reduction atmosphere to obtain the long-afterglow fluorescent powder which can be effectively excited by blue light and high in luminous intensity. The long-afterglow fluorescent powder can satisfy the application requirements of the direct current / alternating current LED, has an actual application valueand is promising in commercial prospect.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
Specific fluorescence labeling method of food-borne probiotics and in-vivo application thereof
InactiveCN108524953AExcellent afterglow performanceGood dispersionIn-vivo testing preparationsLuminescent compositionsFood borneBiological imaging
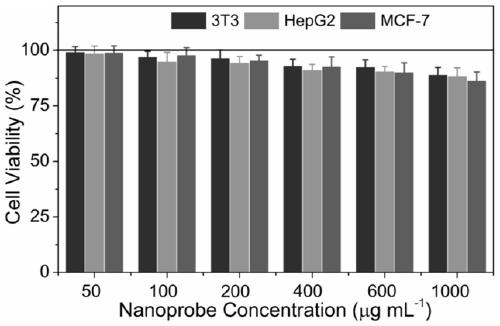
The invention relates to a specific fluorescence labeling method of food-borne probiotics. The method includes following steps: (a), preparing a Cr3+doped gallium zinc germanate long-afterglow fluorescent nano probe having near infrared fluorescence and ultralong-afterglow properties through a solvothermal-high-temperature calcining process; (b), realizing specific fluorescence labeling of the nano probe to target probiotics through antibody surface functionalizing. The method has the advantages that the nano probe prepared by the method has ultrastrong near infrared luminescence, ultralong afterglow life, excellent biocompatibility and structural stability, high grain size uniformity and low toxicity; near infrared fluorescence labeling probiotics in-vivo biological imaging technology developed by the invention can realize nondestructive tracing of distribution of probiotics after entering organisms, and the method is of important significance in developing functional sites of innovative food-borne probiotics and nutriology research concepts.
Owner:NANKAI UNIV
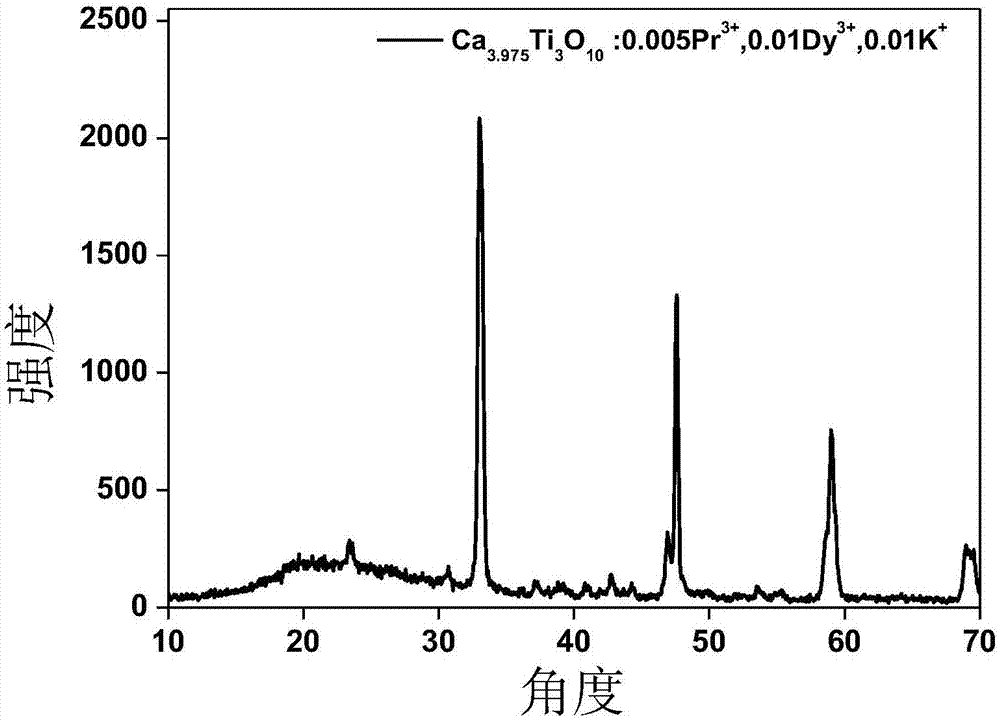
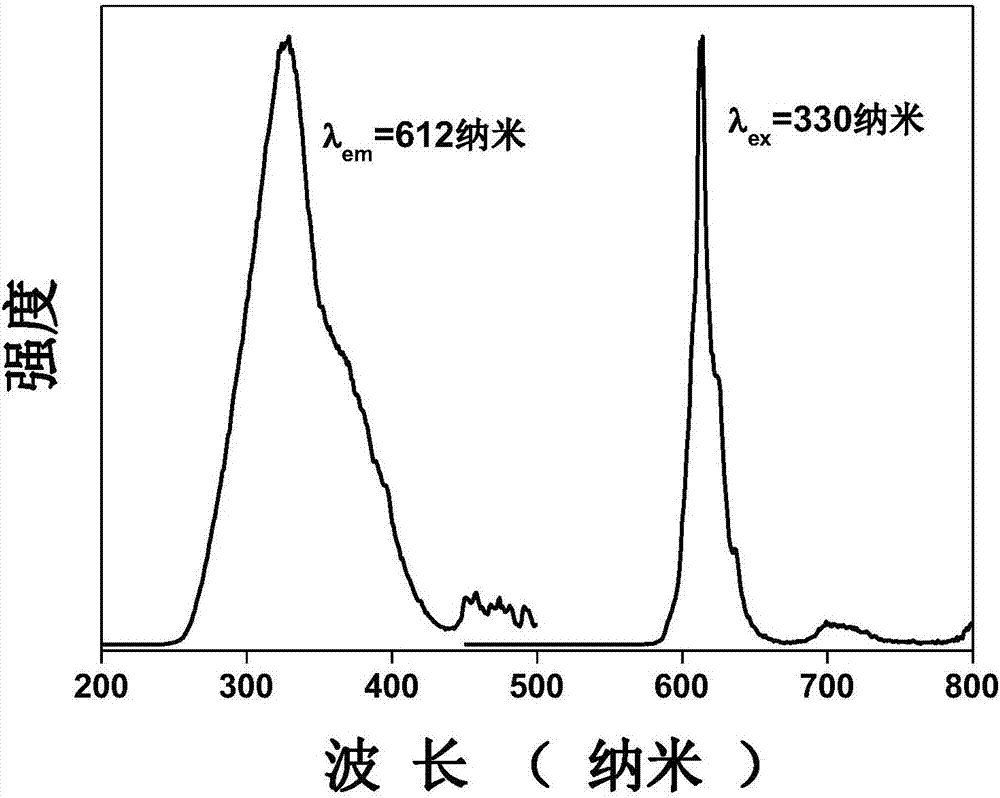
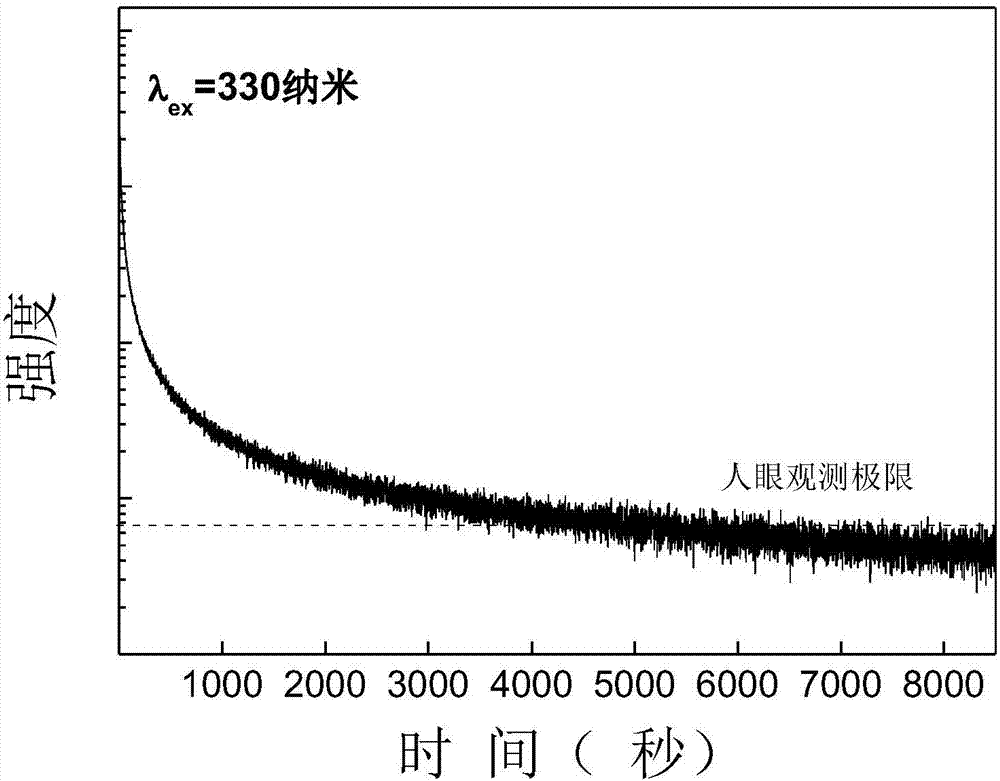
Praseodymium-doped laminated perovskite type red long-lasting phosphor material as well as preparation method and application thereof
InactiveCN107502350ASimple preparation processLow costLuminescent compositionsPhosphorLuminescent material
The invention discloses a praseodymium-doped laminated perovskite type red long-lasting phosphor material and relates to the field of solid light emitting materials. The chemical formula of the material is M4-x-y-zTi3O10:xPr<3+>, yRe<3+>, zA<3+>, in the formula, x is greater than or equal to 0.005 and less than or equal to 0.05, y is greater than or equal to 0 and less than or equal to 0.03, z is greater than or equal to 0 and less than or equal to 0.03, M is one or more elements of Mg, Ca, Sr and Ba, Re is one element of Y, Dy, Lu, Ho, Tm and Yb, and A is one or two elements of Li, Na, K, Zn, B, Al, Ge, Ga, Zr and In. The invention further provides a preparation method of the praseodymium-doped laminated perovskite type red long-lasting phosphor material. The invention further provides application of the praseodymium-doped laminated perovskite type red long-lasting phosphor material. The praseodymium-doped laminated perovskite type red long-lasting phosphor material is low in cost and stable in chemical property.
Owner:WUYI UNIV
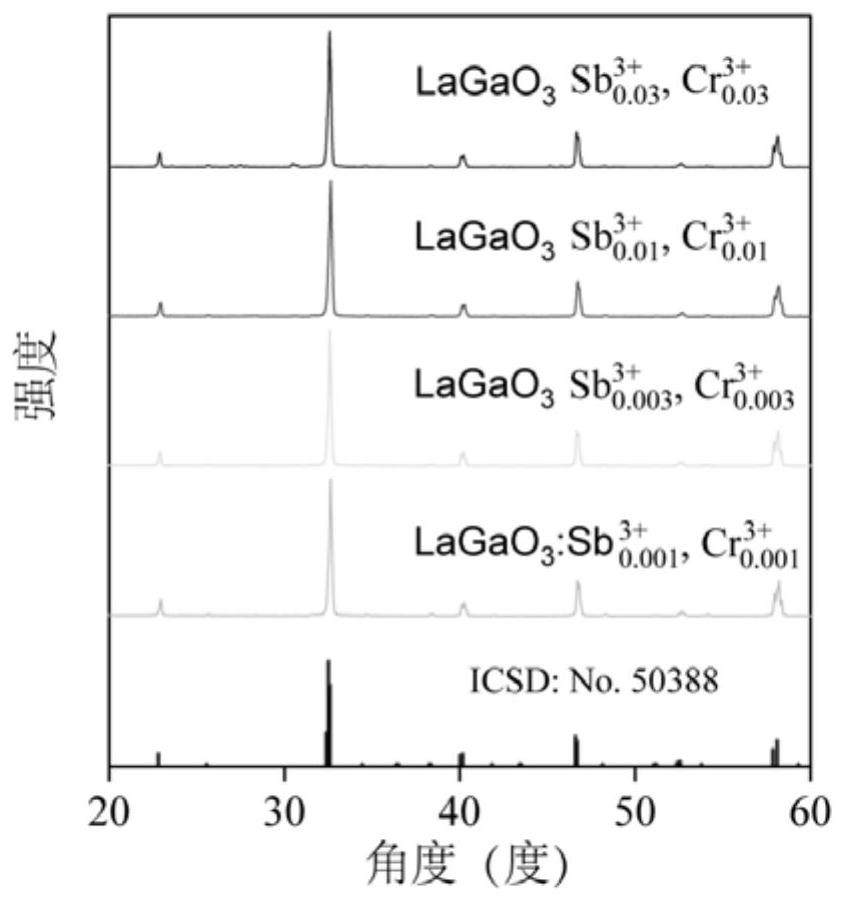
Near-infrared long-afterglow luminescent material, fluorescent probe as well as preparation method and application of near-infrared long-afterglow luminescent material
ActiveCN111892928AExcellent afterglow performanceGood imaging effectColor/spectral properties measurementsFluorescence/phosphorescenceFluoProbesChemical composition
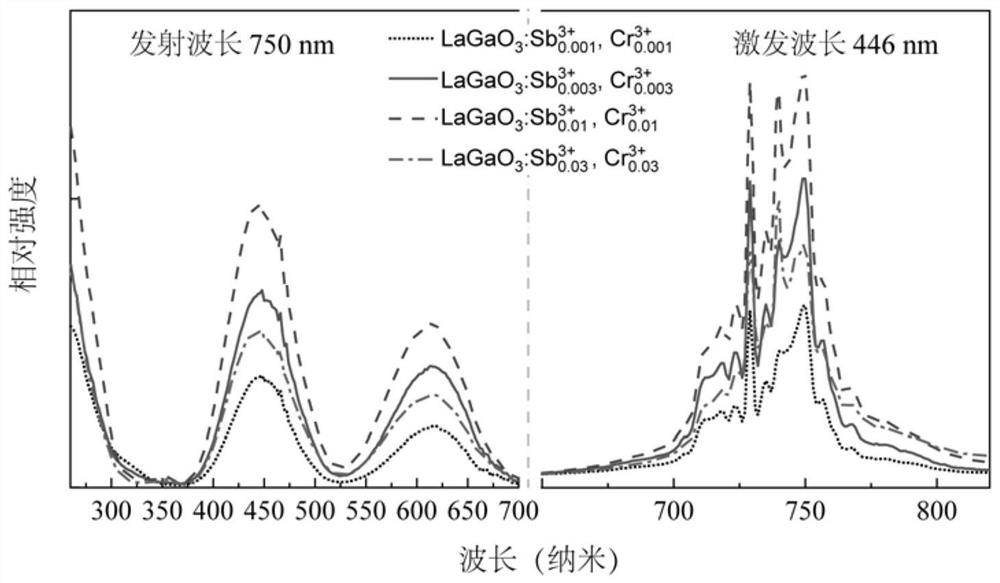
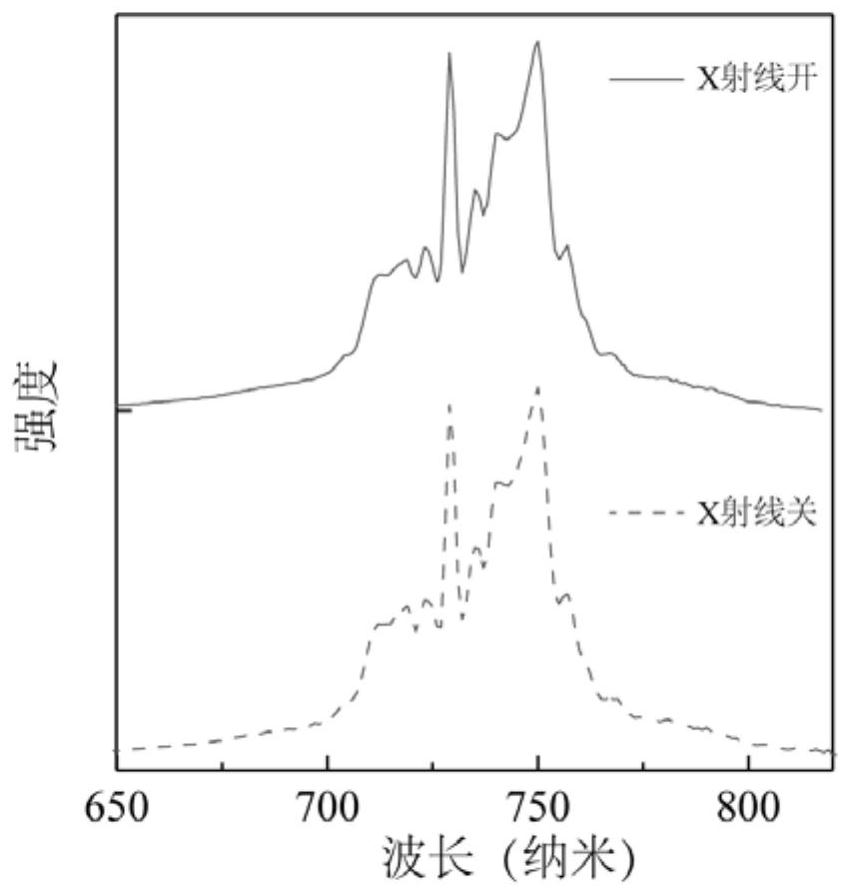
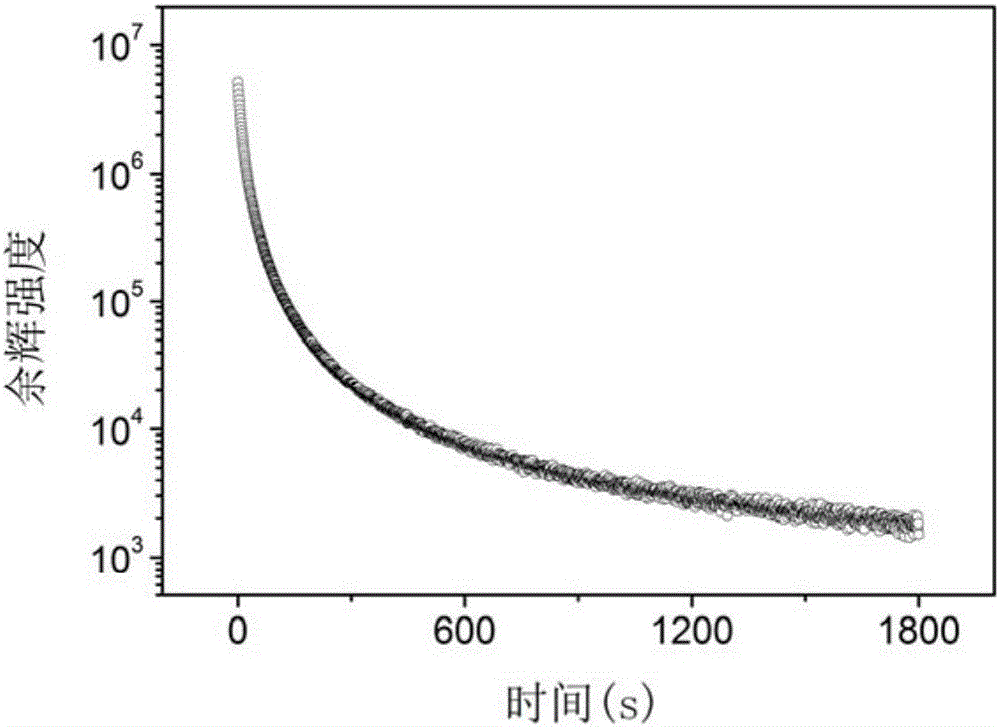
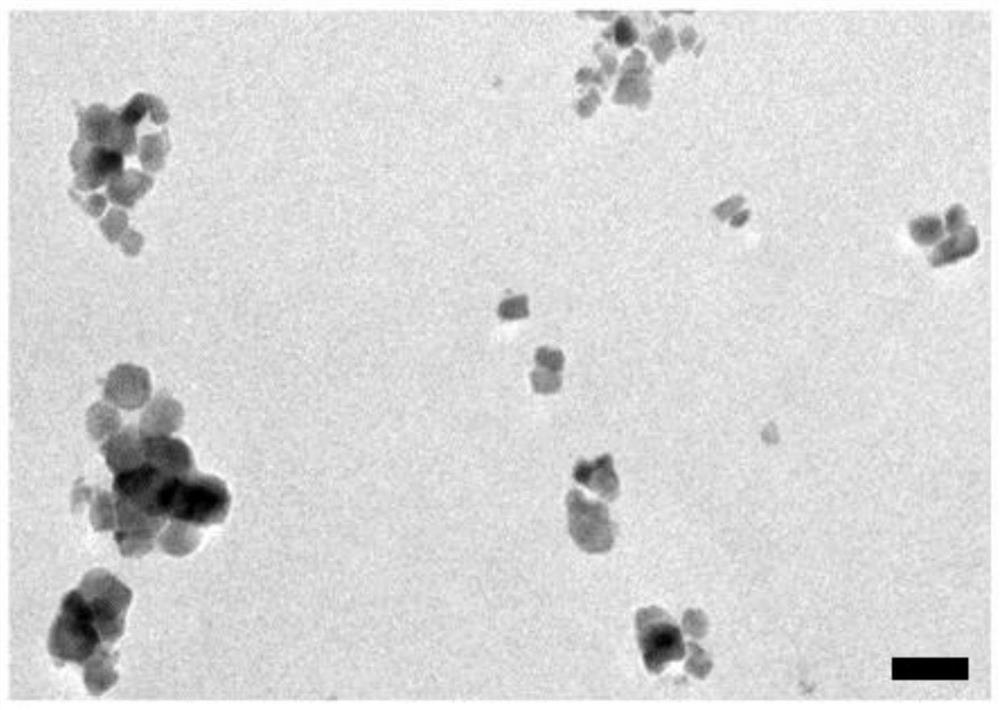
The invention relates to a near-infrared long-afterglow luminescent material, a fluorescent probe as well as a preparation method and application thereof. The chemical composition of the near-infraredlong-afterglow luminescent material is LaGa1-x-yCrxSbyO3, x is greater than or equal to 0.001 and less than or equal to 0.03, and y is greater than or equal to 0.001 and less than or equal to 0.03. According to the near-infrared long-afterglow luminescent material provided by the invention, LaGaO3 is used as a matrix, and ions Cr < 3 + > and Sb < 3 + > are doped to optimize the afterglow performance; the obtained near-infrared long-afterglow luminescent material can be effectively excited by X-rays to generate afterglow emission at 750nm, the afterglow lasting time is as long as 500 hours, and the near-infrared long-afterglow luminescent material has a wide application prospect in photoelectric devices or biological imaging; particularly, the nano-granular near-infrared long-afterglow luminescent material can be prepared into a fluorescent probe for in-vivo imaging, and a better imaging effect can be obtained under the excitation of low-dose X-rays.
Owner:SUN YAT SEN UNIV
Long-lasting phosphor material and preparation method thereof
InactiveCN106147767AWith afterglow propertiesImproved afterglow performanceLuminescent compositionsPhosphorMaterials science
The invention provides a long-lasting phosphor material and a preparation method thereof and relates to long-lasting phosphor materials. The chemical formula of the long-lasting phosphor material is aMIIO.bSc2O3.cZrO2.dCeO2.eMIII2O3.fMIVO2. MII is selected from at least one of Mg, Ca, Sr, Ba and Zn. MIII is selected from at least one of Y, Lu, La, Pr, Nd, Sm, Gd, Tb, Dy, Ho, Er, Tm, Yb, Mn, Ga, In, Sb, Bi and Cr. MIV is selected from at least one of Ge, Sn and Ti. A is larger than or equal to 0.5 and smaller than or equal to 1.5, b is larger than or equal to 0.5 and smaller than or equal to 1.2, c is larger than or equal to 0.001 and smaller than or equal to 0.5, d is larger than or equal to 0.001 and smaller than or equal to 0.2, e is larger than or equal to 0 and smaller than or equal to 0.5, and f is larger than or equal to 0 and smaller than or equal to 0.5. Oxides or corresponding salts of all the elements are weighed according to the mole number ratio of a, b, c and d, and grinding is carried out; grinding is carried out after presintering and cooling; after sintering and smashing are carried out, the long-lasting phosphor material is obtained.
Owner:XIAMEN UNIV
Rare earth ion co-doped near-infrared long-afterglow luminescent nano material as well as preparation method and application thereof
ActiveCN113637476AUniform sizeUniform small sizeNanotechnologyEnergy efficient lightingRare earth ionsPhysical chemistry
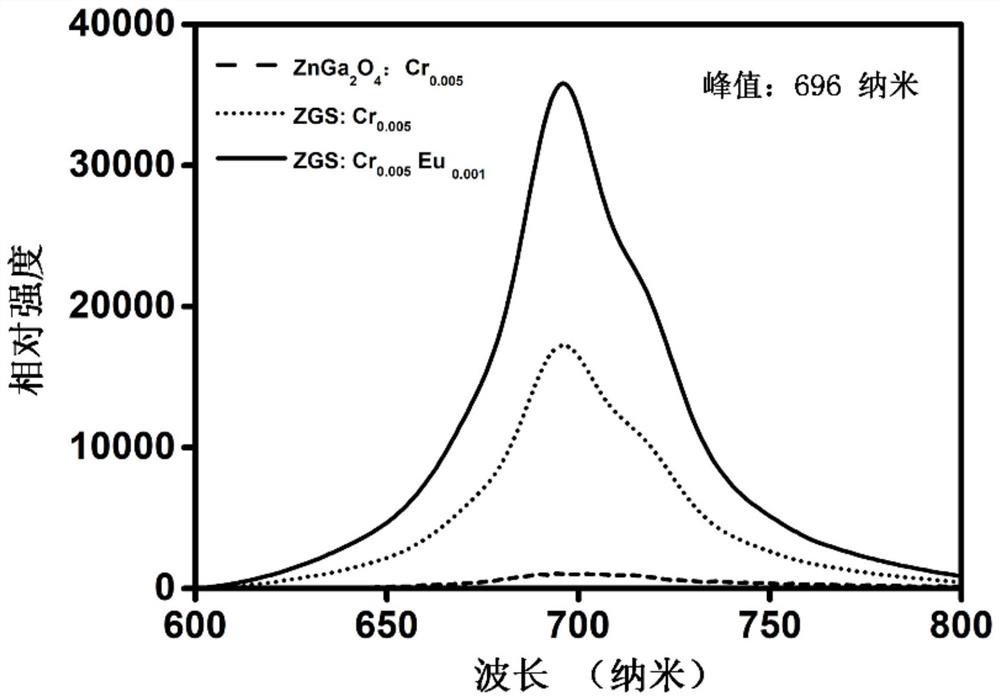
The invention discloses a rare earth ion co-doped near-infrared long-afterglow luminescent nano material as well as a preparation method and application thereof, and belongs to the technical field of near-infrared long-afterglow luminescent materials. The chemical expression of the long-afterglow luminescent nano material is Zn3Ga2Sn2O10: xCr < 3 + >, yEu < 3 + >, wherein Cr < 3 + > and Eu < 3 + > are luminescent central ions, x is more than 0% and less than or equal to 1%, and y is more than 0% and less than or equal to 0.5%. A combustion method is adopted for preparation, acetylacetone metal salt is used as a precursor, the calcination temperature is 850 DEG C, and the calcination time is 2 hours. The synthesized long-afterglow material can be excited by a biological window and has near-infrared first-zone emission, and the afterglow performance of near-infrared first-zone emission is greatly enhanced by adjusting the proportion of doped ions to different values.
Owner:XIAMEN INST OF RARE EARTH MATERIALS
Cyan silicate super-long afterglow luminescent material and preparation method thereof
ActiveCN108949173ANo emissionsThe synthesis method is simpleLuminescent compositionsChemical compositionChemical measurement
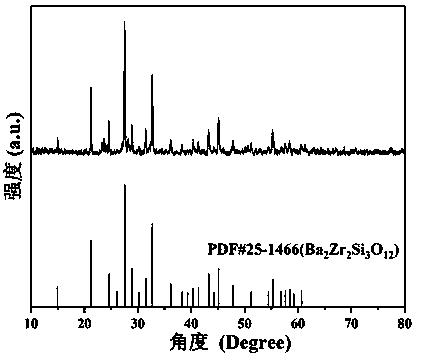
The invention provides a cyan silicate super-long afterglow luminescent material and a preparation method thereof. The chemical expression of the luminescent material is Ba2-m-nZr2-x-yHfxSnySi3O12:Eum, Rn, wherein m is more than 0 but less than or equal to 0.05; n is more than or equal to 0 but less than or equal to 0.05; x is more than or equal to 0 but less than or equal to 2; y is more than orequal to 0 but less than or equal to 2; x+y is more than or equal to 0 but less than or equal to 2; R is one or two of Tb, Ce, Dy, Tm, Nd, Gd, Y, Er, La, Pr, Sm, Yb, Lu and Ho. The cyan silicate super-long afterglow luminescent material is prepared according to the following steps: weighting raw materials according to the stoichiometric ratio of all the chemical compositions in the chemical expression, mixing and grinding, pre-sintering and then calcining under high temperature, cooling following a furnace and then grinding. The luminescent material has high cyan afterglow strength and long afterglow time, has the excellent performances of silicate long afterglow luminescent material as well as the afterglow time equivalent to that of aluminate long afterglow luminescent material, and is simple in preparation method, pollution-free and low-cost.
Owner:LANZHOU UNIVERSITY
Novel white steady persistence afterglow luminescence material and preparing method thereof
ActiveCN107163936AGood color purityExcellent afterglow performanceLuminescent compositionsAfterglowSolid phases
The invention discloses a novel white persistent afterglow luminescence material. The molecular formula of the material is Zn2GeO4, with a main phase structure belonging to a rhombohedral system. The invention is synthesized using a high temperature solid phase method, in which ZnO and GeO2 are grinded, mixed, then calcined, and cooled to obtain the white persistent afterglow luminescence material. Zn2GeO4 in the invention has a band afterglow spectrum across 400 nm to 700 nm after being motivated by a 254 nm ultraviolet lamp, and emits white light observed by naked eyes, and after the light source is removed, the material still emit white persistent afterglow. The color purity of the material is good, the performance of the afterglow is good, and the synthesis method of the material is simple and easy to be achieved.
Owner:BEIJING JIAOTONG UNIV
Germanium stannate long-afterglow luminescent material and preparation method thereof
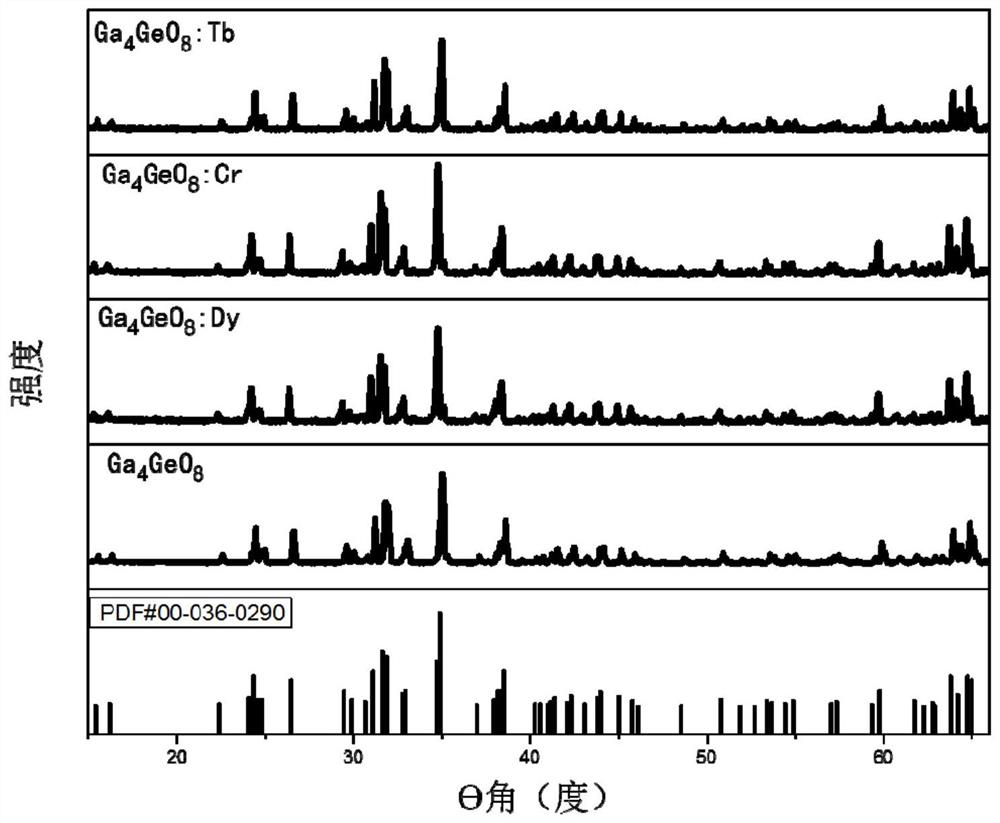
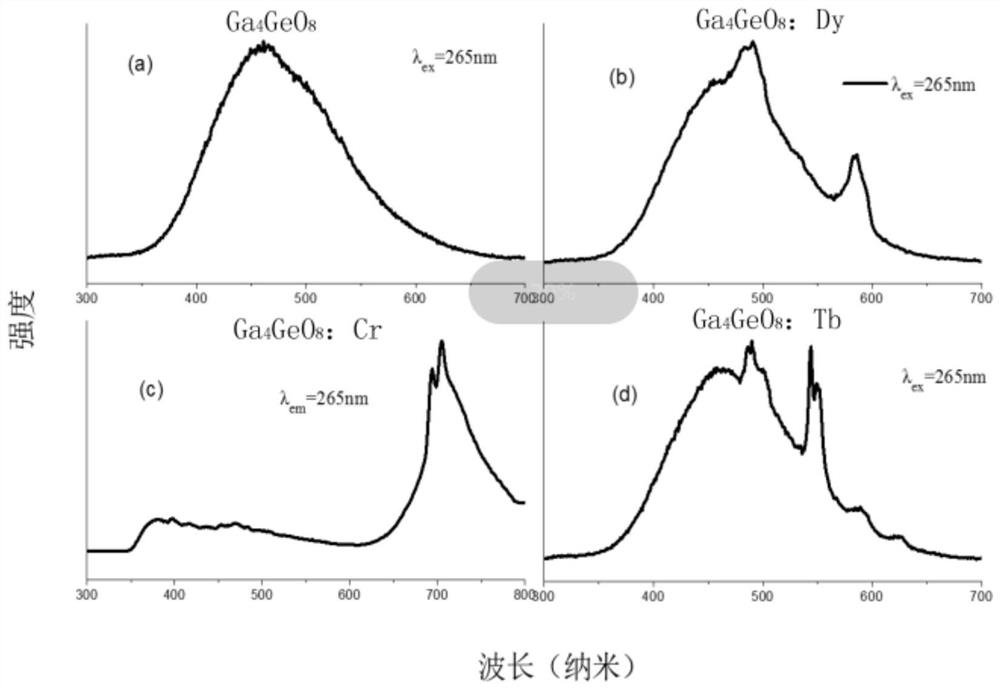
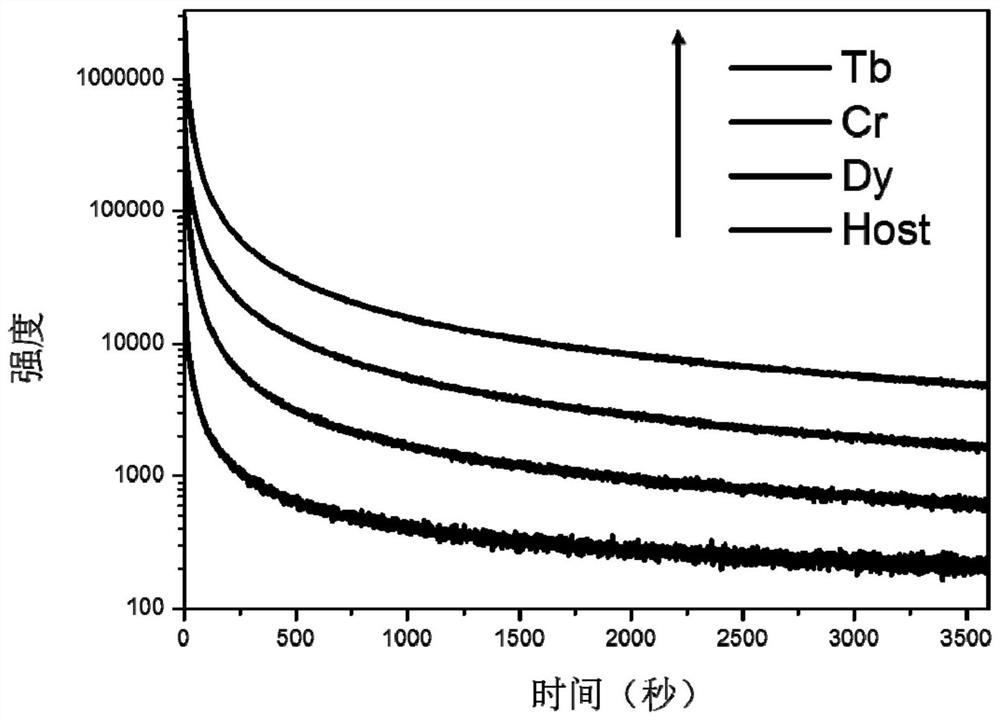
InactiveCN109536165AIncreased range of glow colorsEnhanced luminous color rangeLuminescent compositionsUltrasound attenuationRare earth ions
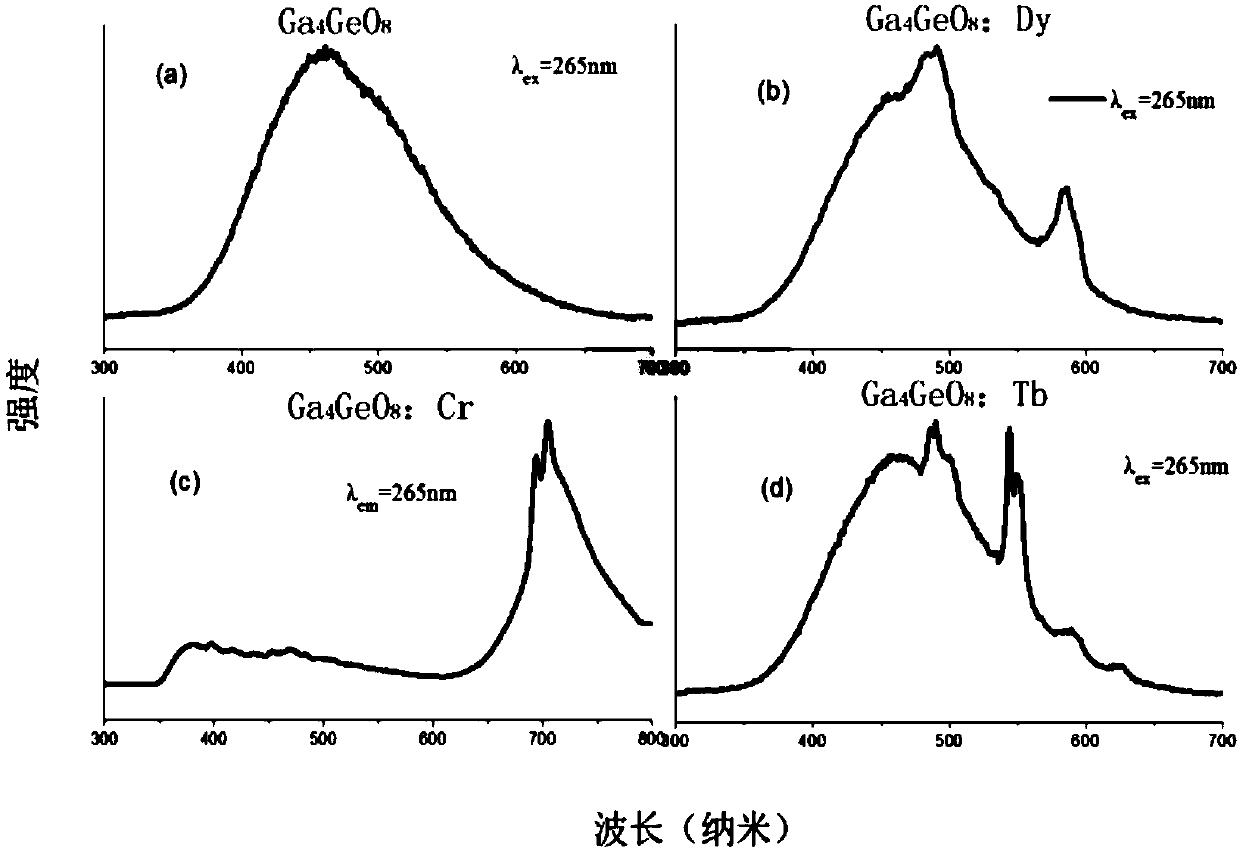
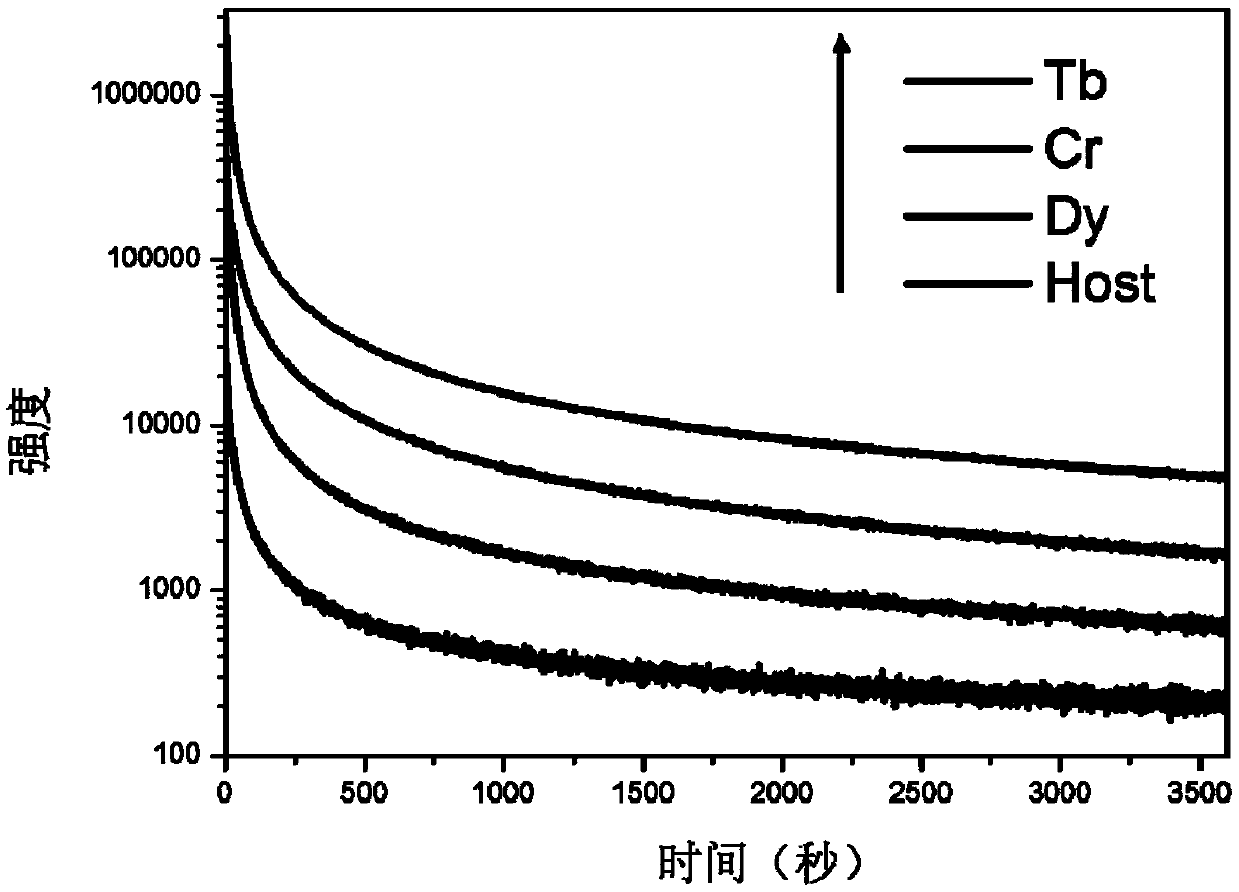
The invention provides a germanium stannate long-afterglow luminescent material and a preparation method thereof and relates to the technical field of an inorganic functional material. According to the invention, gallium germanate is used as a substrate of the long-afterglow luminescent materia. The long-afterglow luminescent material has a following chemical formula: Ga4GeO8:xLn<3+>, wherein Ln is a trivalent rare earth ion and / or a trivalent transition metal ion and x is a molar fraction. The long-afterglow luminescent material provided by the invention has the advantages that afterglow attenuation is slower, afterglow emission time can last for hours, light with different colors and various afterglow colors can be formed by doping different ions, and application prospect is good and application field is extensive.
Owner:XIAMEN UNIV OF TECH
Near-infrared high-afterglow material and preparation method thereof
ActiveCN107057697AImprove performanceExcellent afterglow performanceLuminescent compositionsAlcoholSolvent
The invention relates to a near-infrared high-afterglow material and a preparation method thereof and aims to solve the technical problem that existing near-infrared long-afterglow materials are weak in afterglow intensity and short in afterglow time. A molecular formula of the near-infrared high-afterglow material is (Zn1-xCax)3(Ga0.995Cr0.005)2G22O10, wherein x is greater than or equal to 0.01 and smaller than or equal to 0.03. The preparation method includes: well mixing zinc oxide, calcium oxide, gallium oxide, chromium oxide and germanium oxide according to a chemical dosage ratio, adding a mixture into water, boiling the water, and adding nitric acid to dissolve the oxides to obtain a solution; mixing the solution with a mixed solution of water, alcohol and oleic acid to obtain a mixed solution, and performing hydrothermal reaction to obtain a solid-phase product; subjecting the solid-phase product to pre-sintering and high-temperature sintering to obtain the near-infrared high-afterglow material. The near-infrared high-afterglow material can be used in the technical field of bioluminescent imaging.
Owner:HARBIN INST OF TECH
Rare earth doped long afterglow material and preparation method and application thereof
ActiveCN113502162AExcellent afterglow performanceOvercome the problem of not being able to simultaneously enhance near-infrared dual emissionsLuminescent compositionsPhysical chemistryMaterials science
The invention provides a rare earth doped long-afterglow material and a preparation method and application thereof, the rare earth doped long-afterglow material is represented by a chemical formula MgGeO3: aMn < 3 + >, bYb < 3 + > and cY < 3 + >, Mn < 3 + > and Yb < 3 + > are luminescent central ions, Y < 3 + > is a sensitizing ion, wherein 0.01 mol% < = a < = 5mol%, 0.25 mol% < = b < = 3mol%, and 0.25 mol% < = c < = 3 mol%; by introducing Y < 3 + > for doping and controlling the molar amount of luminescent central ions and sensitized ions, the finally obtained rare earth doped long afterglow material has excellent luminescent effects in a near-infrared first region and a near-infrared second region at the same time; the problem that a long-afterglow material prepared by a traditional method cannot meet the requirement that a near-infrared region has double emission peaks at the same time is solved.
Owner:JIANGXI INST OF RARE EARTHS CHINESE ACAD OF SCI
A preparing method of a nanometer near-infrared long-afterglow material
InactiveCN108949174AHas a synthesis temperatureExcellent afterglow performanceLuminescent compositionsNanoparticleCentrifugation
The invention relates to a long-afterglow luminescent material, and particularly provides a preparing method of a nanometer near-infrared long-afterglow material. The method includes mixing Na2CO3, Ga2O3 and GeO2 according to a stoichiometric ratio; grinding and calcining the mixture to obtain Na2Ga<2(1-x-y)>Ge<x>O<4-x> powder; preparing a suspension from the powder; mixing the suspension with Zn(CH3COO)2 and Cr(CH3COO)3 in a stoichiometric ratio to perform ion exchange to obtain a precursor of the near-infrared long-afterglow material; and calcining the precursor, or transferring the precursor to a reaction kettle with a polytetrafluoroethylene liner, performing a hydrothermal reaction and centrifugation, then washing a product with water and ethanol alternatively, drying a product and calcining the product to obtain the nanometer near-infrared long-afterglow material. The method is simple, the calcining temperature is relatively low, and the prepared nanoparticles have good afterglowperformance and are prone to large-scale promotion and application.
Owner:XIAMEN UNIV
Intracellular fluorescence labeling method of food-derived probiotics and in-vivo application thereof
InactiveCN109939244AExcellent afterglow performanceGood dispersionDiagnostic recording/measuringSensorsBiological imagingA-DNA
The invention relates to an intracellular fluorescence labeling method of food-derived probiotics. The method comprises the following steps that a, a long-afterglow nano-luminescent material is modified by using a DNA; b, a probiotic competence is prepared; c, a DNA coated nano fluorescent probe is electrically transferred to the probiotics; d, the nano fluorescent probe is orally taken into a mouse body for imaging. The method has the advantages that the method for using the nano-luminescent material for intracellular fluorescence labeling is achieved for the first time, complex surface functionalization and antibody modification of a fluorescent material are not needed, the steps are simple and convenient, the cost is low, and compared with surface marking, the method has better stability and marking reproducibility. According to a near-infrared fluorescence labeling probiotic body in-vivo biological imaging technology developed through the method, the long-afterglow nano-luminescentproperty inside thalli can be used, metabolic behaviors and distribution conditions of probiotic bodies in a biological body can be monitored losslessly in situ in real time, and an innovative research means is provided for functional sites and nutriology of the food-derived probiotics.
Owner:NANKAI UNIV
A kind of germanate long afterglow luminescent material and preparation method thereof
InactiveCN109536165BIncreased range of glow colorsEnhanced luminous color rangeLuminescent compositionsChemical physicsRare earth ions
The invention provides a germanium stannate long-lasting luminescent material and a preparation method thereof, and relates to the technical field of inorganic functional materials. The long afterglow luminescent material of the present invention is based on gallium germanate, and the long afterglow luminescent material has the following chemical formula: Ga 4 GeO 8 :xLn 3+ , wherein, Ln is a trivalent rare earth ion and / or a trivalent transition metal ion; x is a mole percentage. The long afterglow luminescent material of the present invention has a slow afterglow attenuation, and the afterglow emission time can last for several hours, and can form a variety of different colors of luminescence and afterglow colors through different doped ions, and has good application prospects and a wide range of application fields .
Owner:XIAMEN UNIV OF TECH
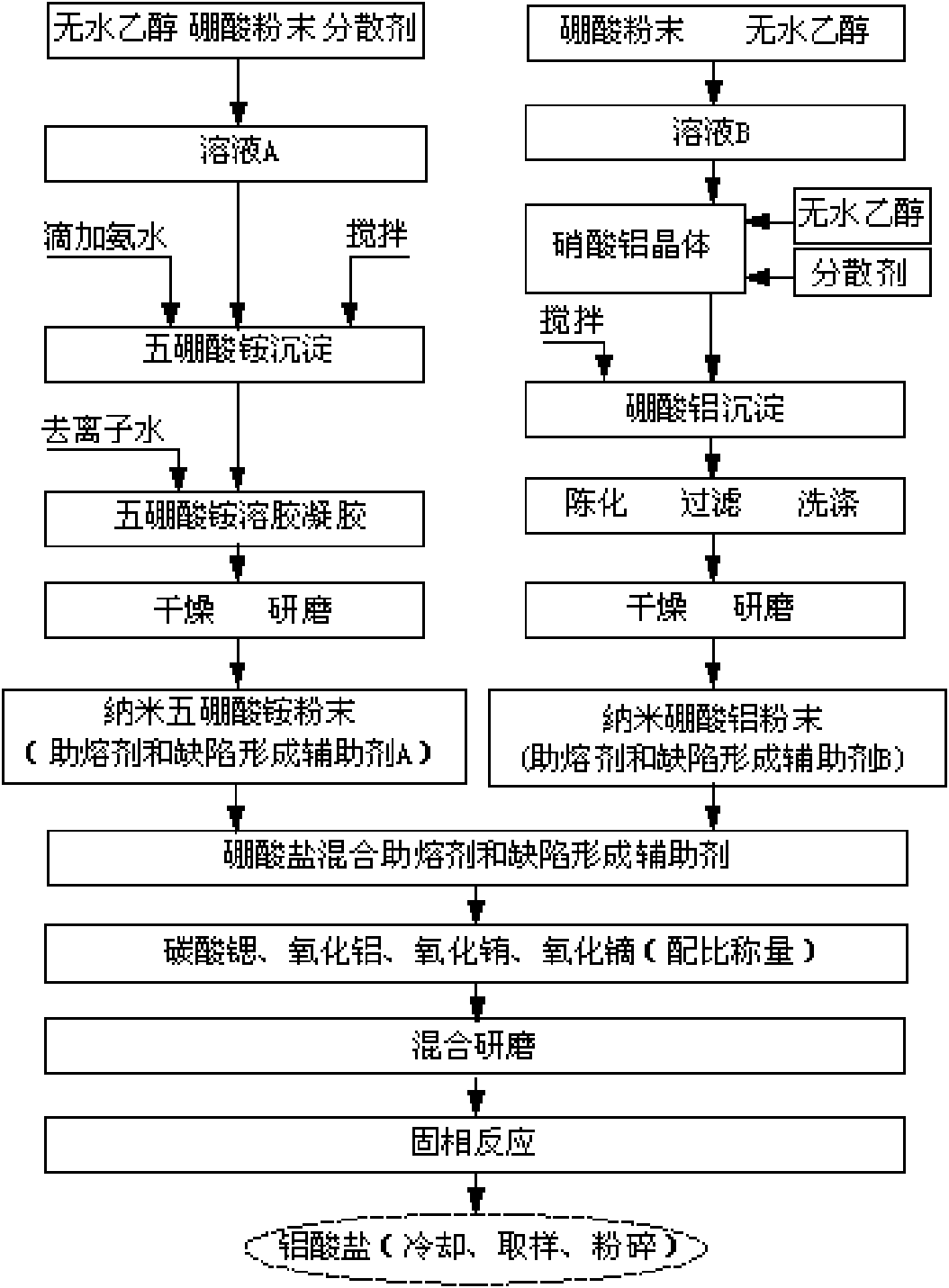
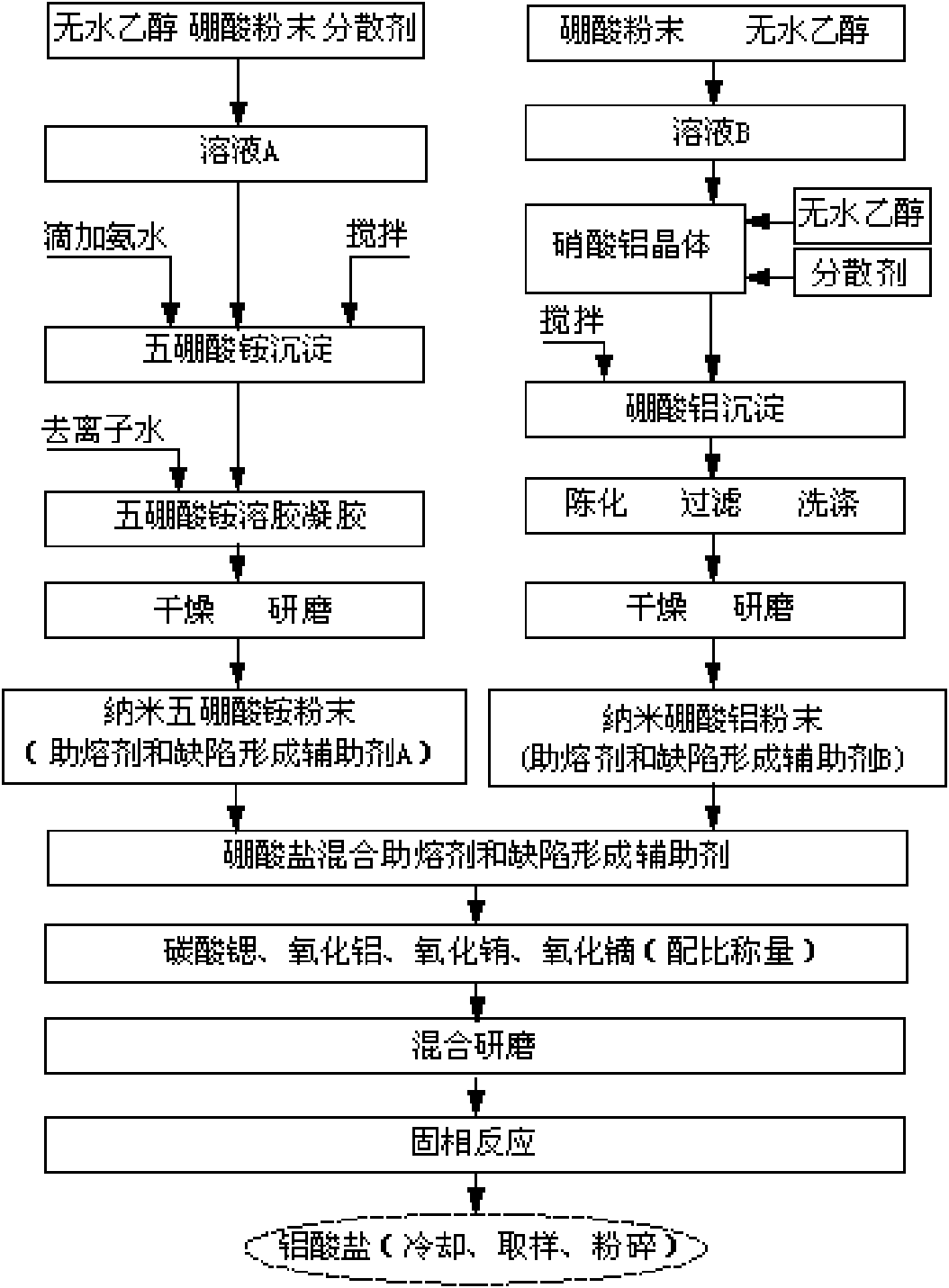
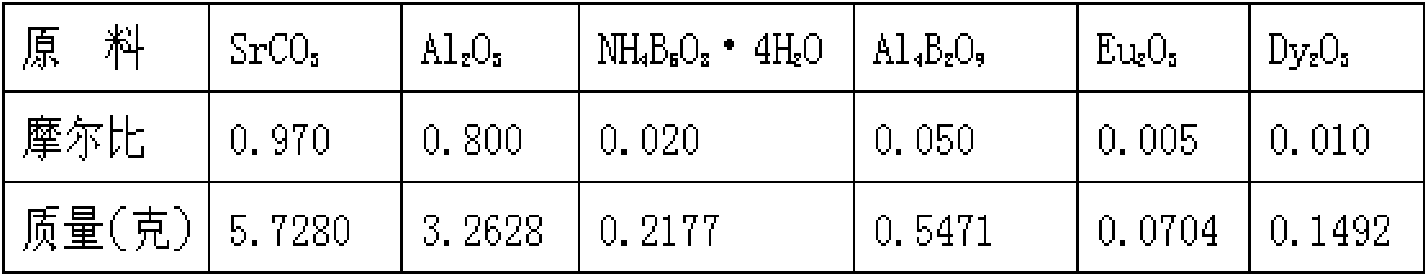
Method for preparing strontium aluminate long-persistence luminescent materials based on nanometer fusing assistants
InactiveCN101768439AOvercome the defects of high reaction temperature, long reaction time and high energy consumptionSolve the problem of poor application effectChemical industryLuminescent compositionsReaction temperaturePrinting ink
The invention provides a method for preparing strontium aluminate long-persistence luminescent materials based on nanometer fusing assistants. The invention has the technical scheme that the method comprises the following steps: simultaneously preparing a nanometer ammonium pentaborate powder fusing assistant A and a nanometer aluminium borate powder fusing assistant B by a liquid phase method; then, obtaining boron of a mixed fusing assistant and a defect forming assistant through the introduction of the fusing assistant A and the fusing assistant B; and finally, using solid-phase reaction for preparing the long-persistence luminescent materials at the low reaction temperature. The invention overcomes the defects of overhigh temperature required by the reaction, high cost of equipment used in the reaction, high consumption of energy required in the synthesis process, poor luminescent performance of products and the like when the existing solid-phase method is used for preparing the strontium aluminate long-persistence luminescent materials. At the same time, the invention also solves the problems that the traditional products only pursue the persistence time, so the fusing assistant with high boron content is added, the strontium aluminate reaction products can easily obtain the excessive sintering, and the application effect of the pulverized strontium aluminate reaction products is poor. The luminescent materials of the invention are mainly applied to the fields of safety passage display, luminescent printing ink, luminous lighting and light detection, and are also usedfor novel energy-saving LED lamps with persistence.
Owner:XIANGTAN UNIV
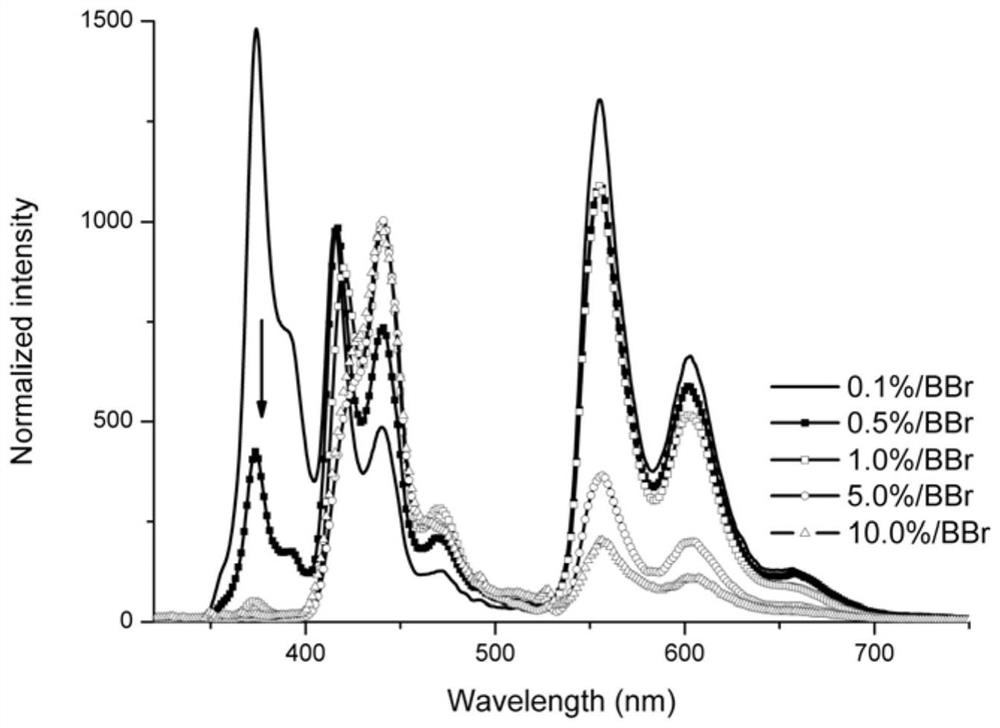
A pure organic material with strong yellow afterglow and its preparation method and application
ActiveCN110092742BSimple structureThe synthesis process is simpleOrganic chemistry methodsLuminescent compositionsQuantum efficiencySimple Organic Compounds
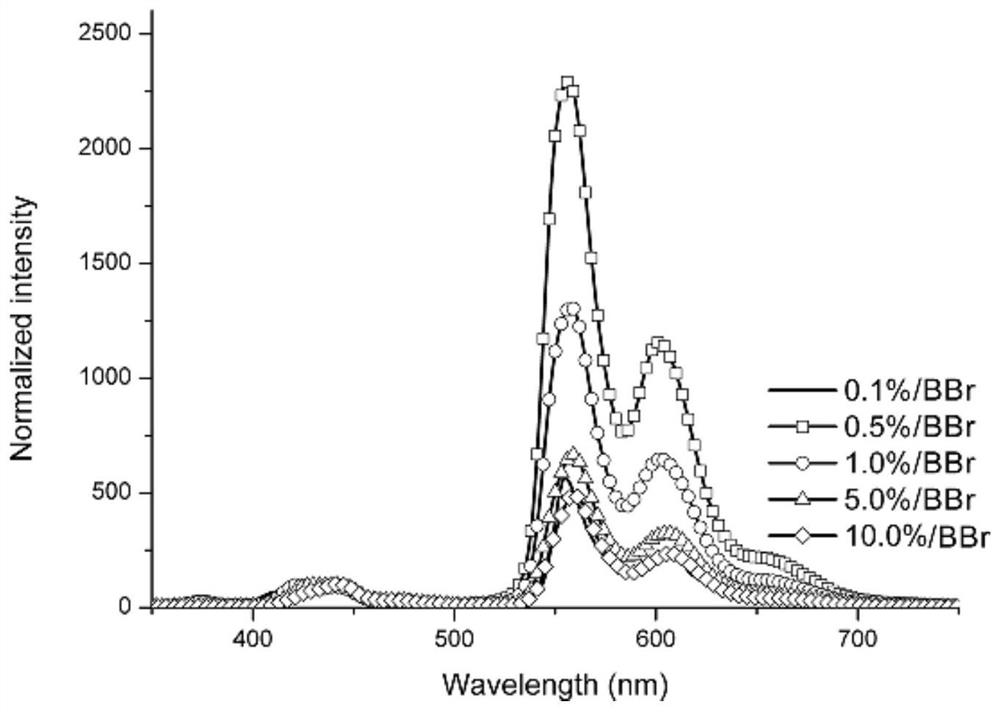
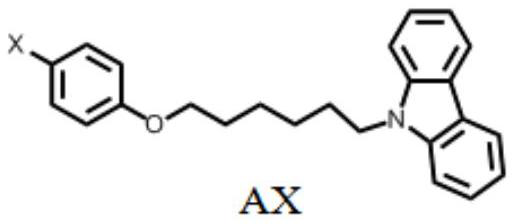
The invention discloses a pure organic material with strong yellow afterglow luminescence and its preparation method and application. The organic compound AX and BBr are mixed, the molar content of the AX is 80%-99.9%, and the BBr The molar content is 0.1% to 20%; when BBr is doped into ABr at a concentration of 0.1% to 10%, the obtained material emits white light under the excitation of ultraviolet light, and the phosphorescence quantum efficiency is between 12 and 39%. The life span is between 90 and 168ms. When BBr is doped into Al at a concentration of 0.1%-10%, the obtained material emits yellow light under the excitation of ultraviolet light, the phosphorescence quantum efficiency is between 19-51%, and the phosphorescence lifetime is between 9-30ms. The preparation method of the material involved in the invention is simple, and due to the high phosphorescence quantum efficiency, the material will have wide application in the fields of biological imaging, anti-counterfeiting marks and the like.
Owner:TIANJIN NORMAL UNIVERSITY
Ytterbium-containing near-infrared ultra-long-afterglow gallate luminescent material and preparation method thereof
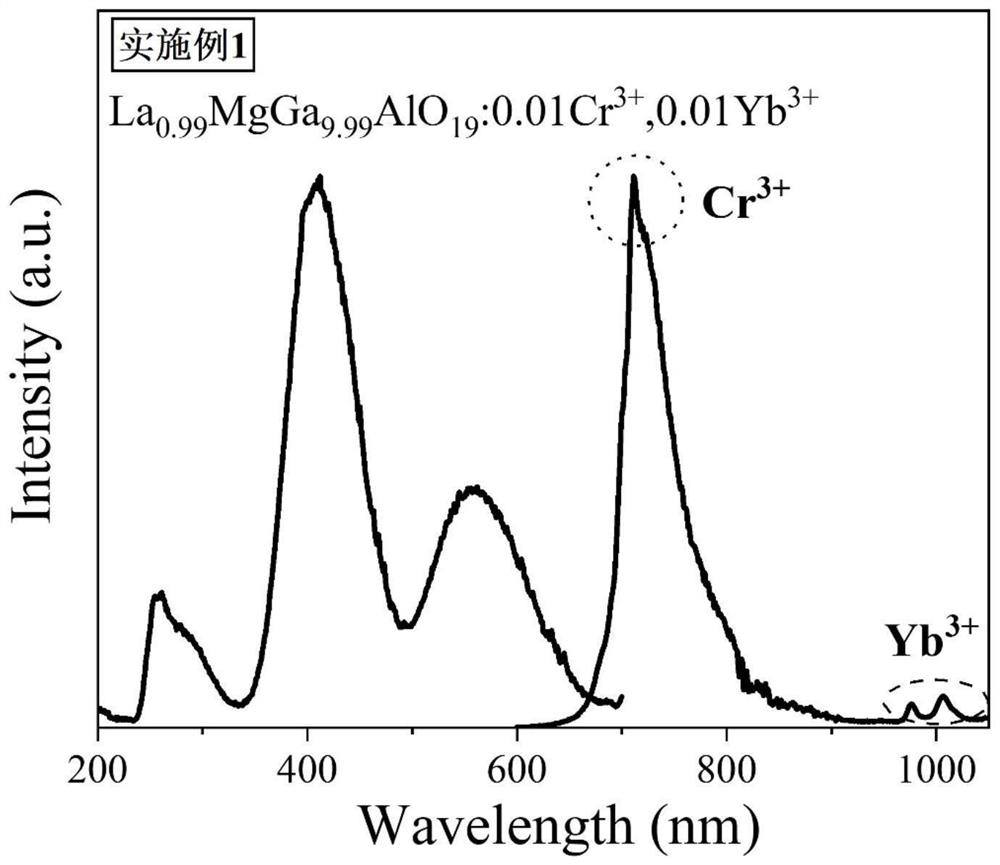
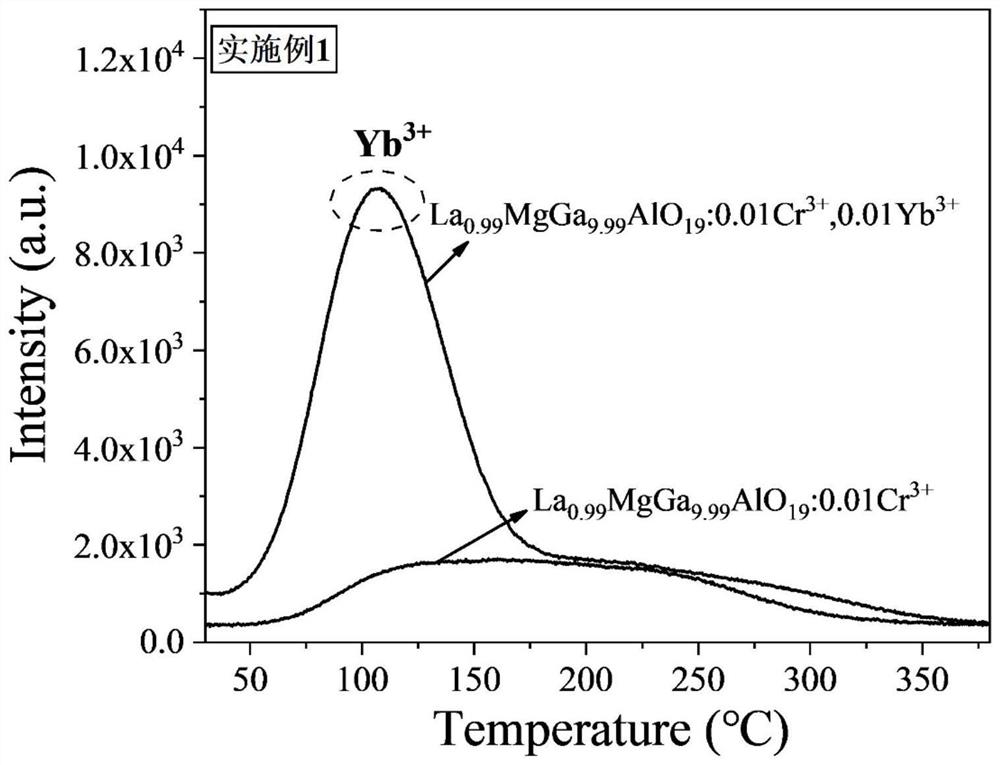
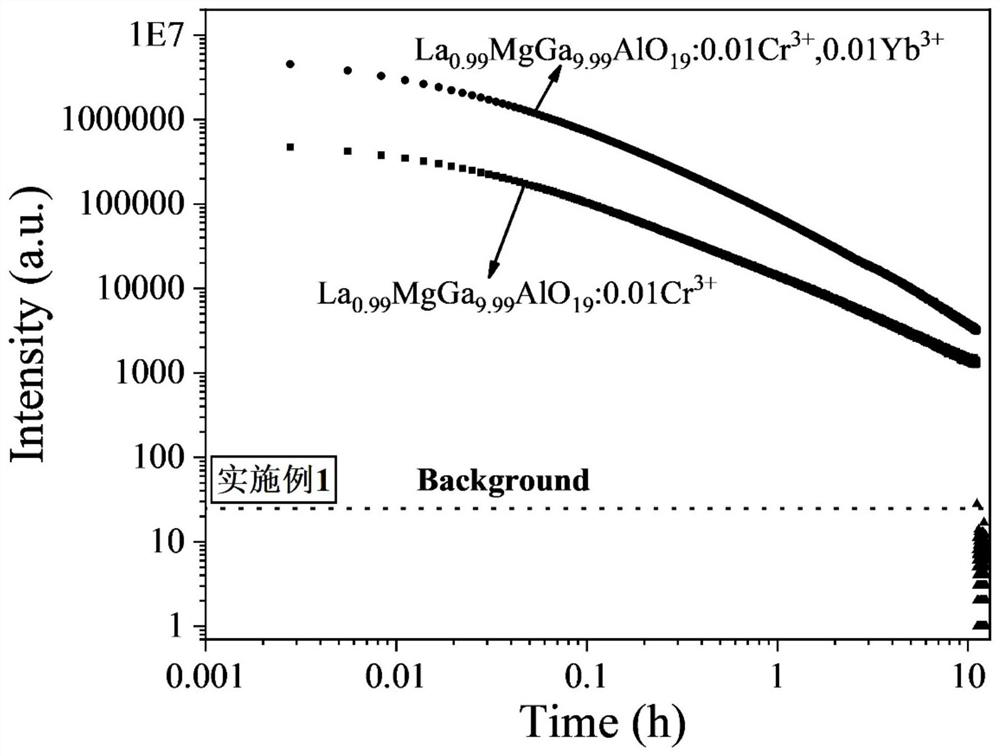
ActiveCN113088286AWide excitation spectrumExcellent afterglow performanceLuminescent compositionsThermoluminescenceUltraviolet lights
The invention discloses an ytterbium-containing near-infrared ultra-long-afterglow gallate luminescent material and a preparation method thereof, belonging to the technical field of near-infrared luminescent materials. The chemical general formula of the material is La<1-y>AGa<11-x-z>Al O<19>: xCr<3+> and yYb<3+>, wherein A is one or two selected from the group consisting of elements with valence of +2, namely Zn and Mg; x is more than or equal to 0.0001 and less than or equal to 1; y is more than or equal to 0.0001 and less than or equal to 1; and z is more than or equal to 0 and less than or equal to 5. Yb<3+> is used as an electron trap, a new thermoluminescence peak appears in a range of 50-150 DEG C after Yb<3+> is added, afterglow intensity and afterglow time are remarkably improved, and Cr<3+> is used as a luminescence center. The near-infrared ultra-long-afterglow luminescent material is of a hexagonal structure and has a space crystal structure which is the same as the space crystal structure of LaMgGa<11>O<19>, and the space group of the material is P63 / mmc. The excitation wavelength range of the material is 250-700 nm, and the emission wavelength range of the material is 600-1100 nm; the material has ultra-long afterglow decay time of 10 hours or more after being irradiated by ultraviolet light for 5 minutes, and the afterglow performance of the material is superior to the afterglow performance of a current commercial ZnGa<2>O<4>: Cr<3+> near-infrared long-afterglow luminescent material. Experiments show that the material has wide application prospects in the aspects of counterfeiting prevention, military affairs, biological imaging, optical information storage and the like. The material is simple to prepare, can be formed by one-time sintering and is easy to technically popularize.
Owner:UNIV OF SCI & TECH BEIJING
Rare earth halosilicate red long-afterglow phosphor, and preparation method thereof
InactiveCN102226086BIncrease surface areaUniform and stable heatingLuminescent compositionsRare earth ionsPhosphor
Owner:LONGNANXIAN SHUNDE MINGHUI FLUORESCENT MATERIAL
Silicate green long afterglow material and preparation method thereof
InactiveCN101575510BShine wellExcellent afterglow performanceChemical industryLuminescent compositionsChemical industryFurnace temperature
The invention relates to a silicate green long afterglow material. The structural formula of a compound of the silicate green long fluorescent lag material is M1-yZn2-xSi2O7:xMn,yRe,zH3BO3, wherein the ratio of (M)O to ZnO to SiO2 is 1:2:2; x, y and z refer to molar coefficient ratio; Mn is an activator; Re is a coactivator; and H3BO3 is an auxiliary solvent. The invention adopts a technical proposal: adopting silicate as a substrate, single Mn<2+> ions as the activator, and doped ion Re as the coactivator, and fully mixing the raw materials with the auxiliary solvent in proportion; and igniting the mixture for 2 to 4 hours in a high-temperature furnace at the temperature of between 1,000 and 1,300 DEG C in a reducing atmosphere or in the air, cooling the obtained product along the furnace temperature, and taking out the obtained product. The silicate green long afterglow material overcomes the defect of a few varieties of the prior long afterglow material and particularly overcomes the defects of a few luminous colors, weak after glow brightness, poor water resistance and stability, and the like, and is suitable for passive display and energy-saving illumination in the fields of traffic, building, chemical industry, mine, household electrical appliance and the like.
Owner:XIANGTAN UNIV
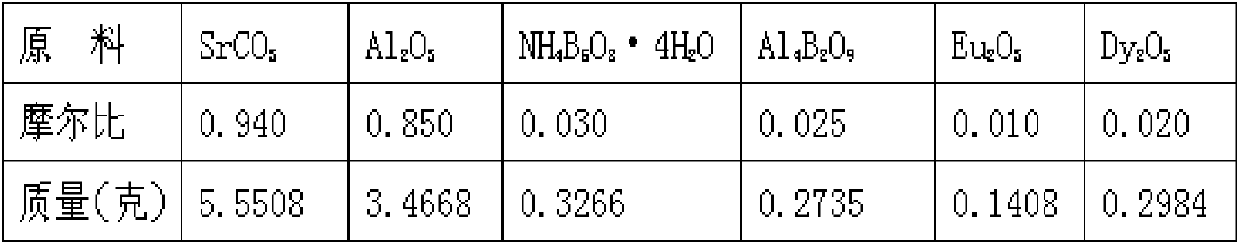
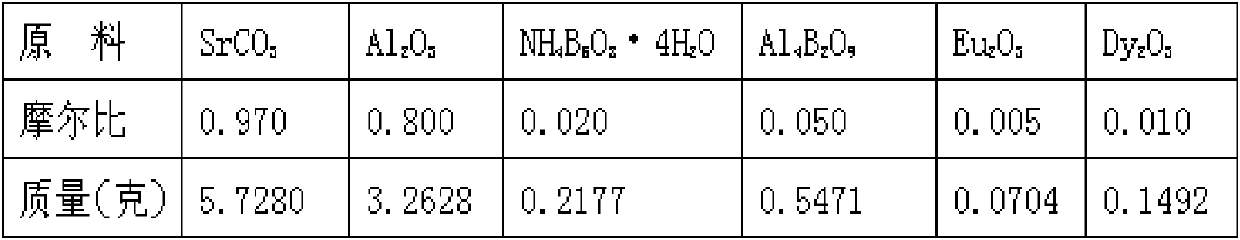
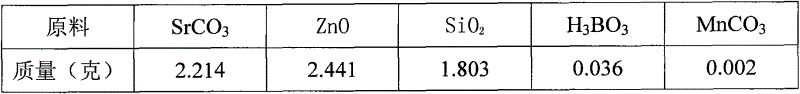
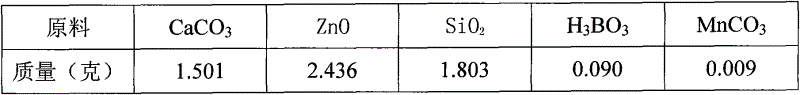
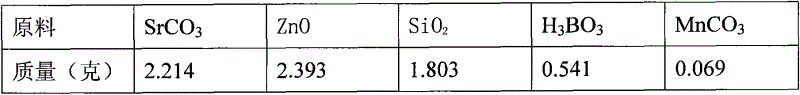
A high-performance europium, dysprosium co-doped strontium aluminate long-lasting phosphor and preparation method thereof
ActiveCN108504353BExcellent afterglow performanceHigh luminous intensityLuminescent compositionsPhysical chemistryDysprosium
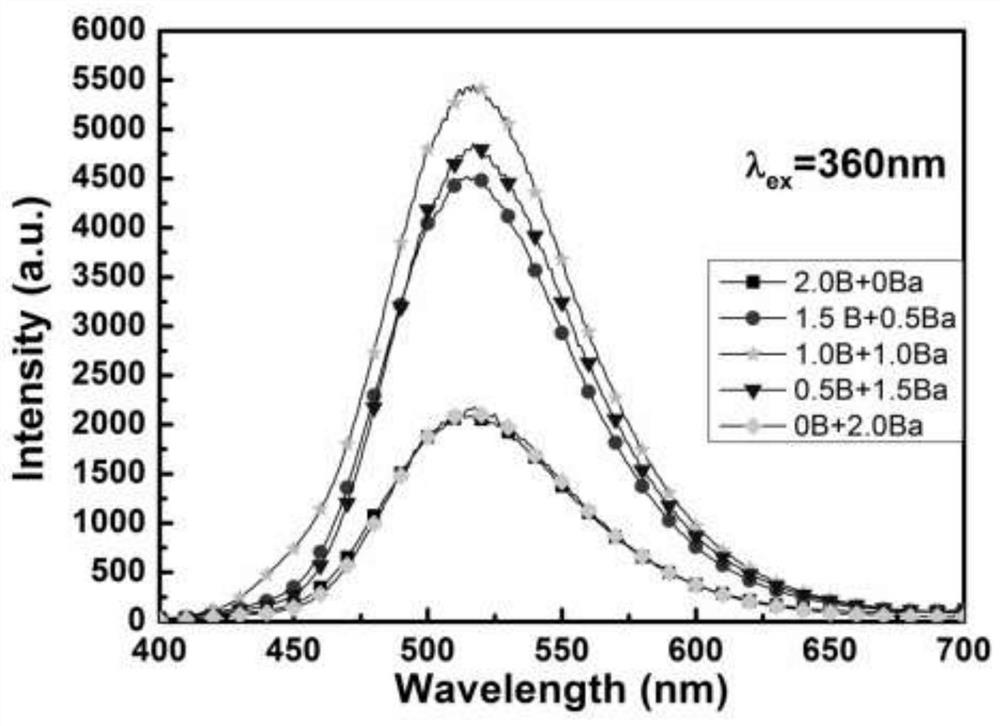
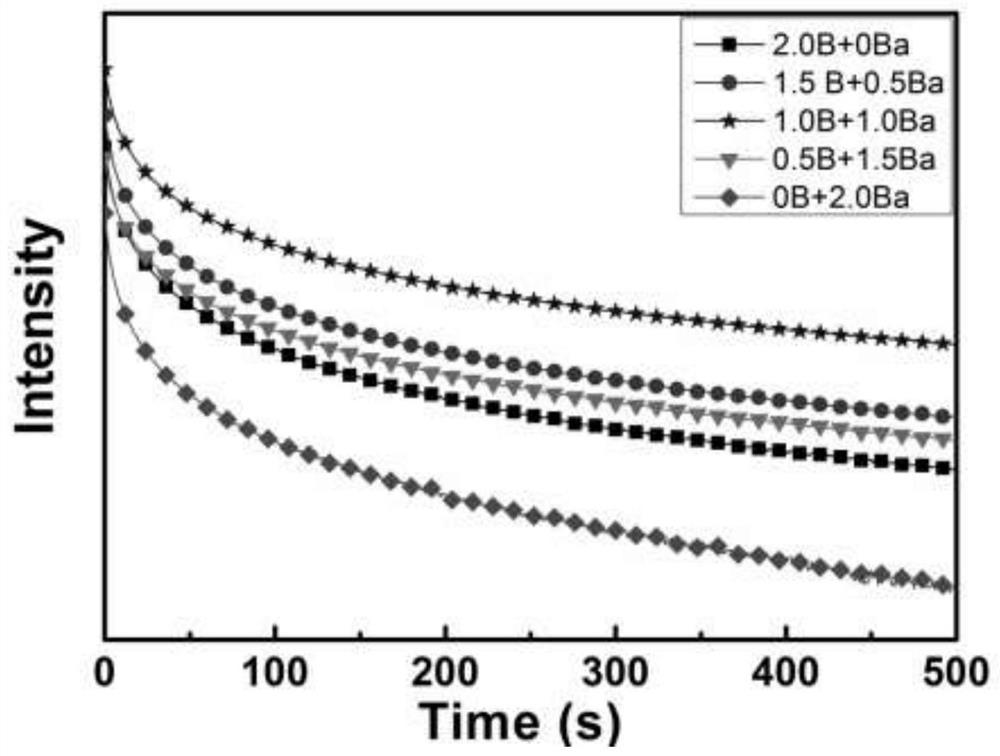
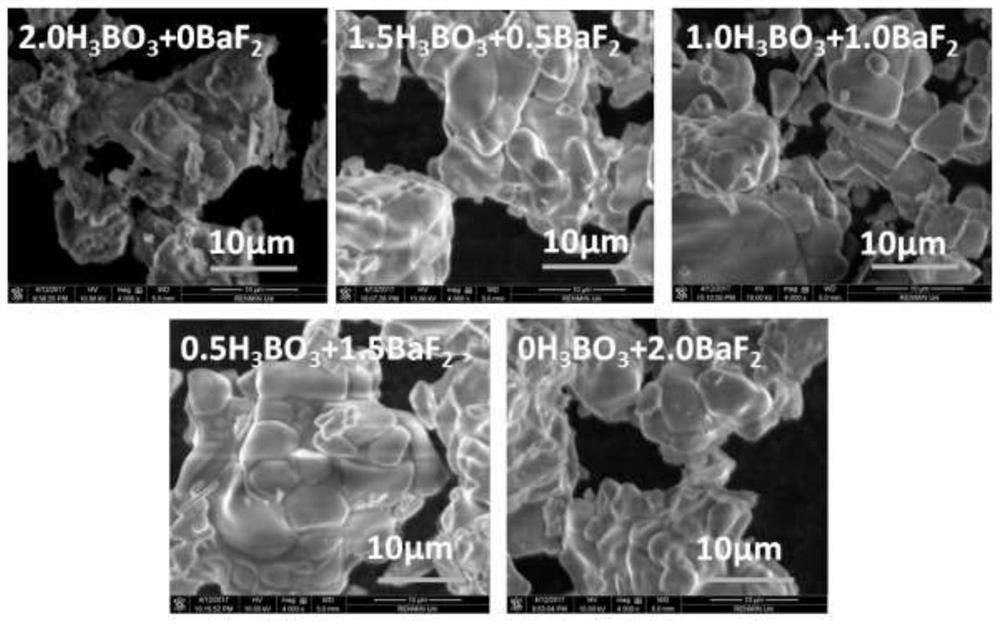
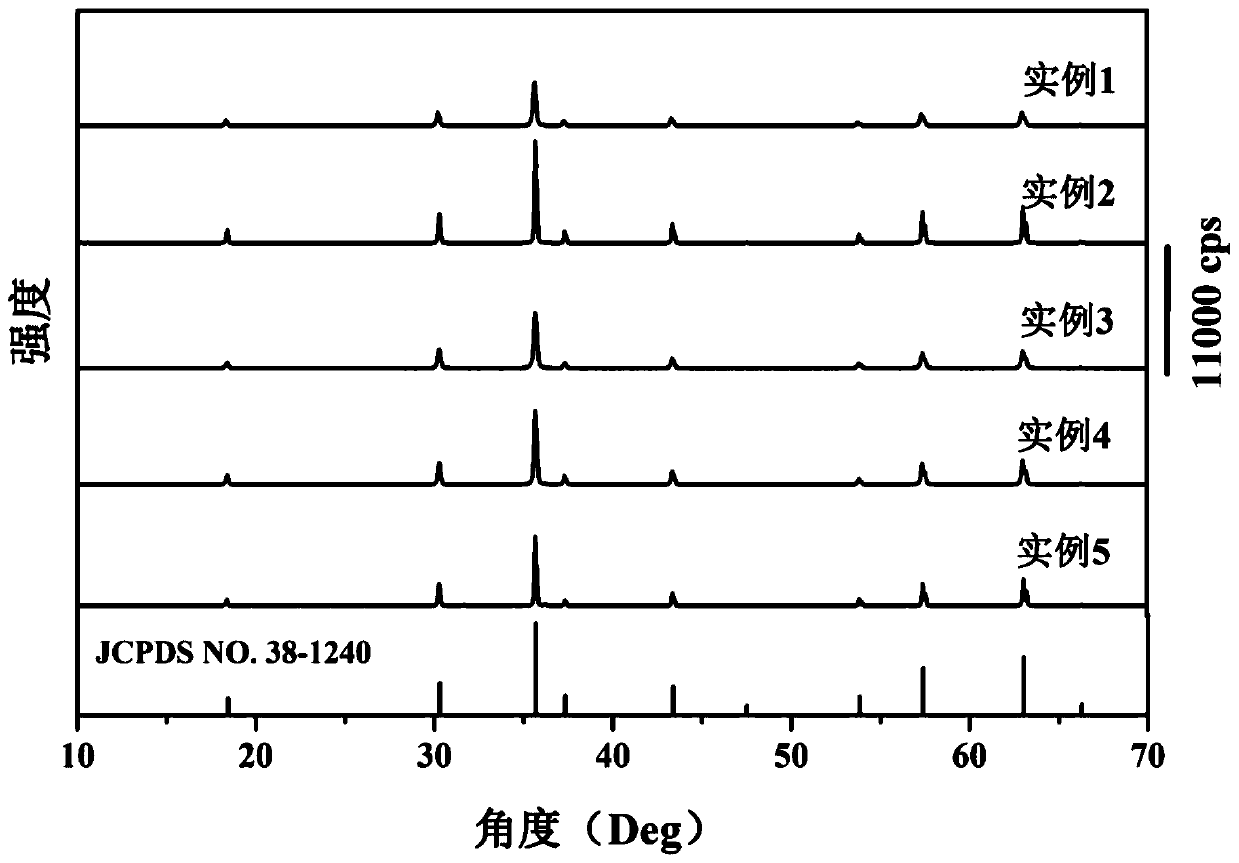
The invention discloses a method for preparing high-performance europium and dysprosium co-doped strontium aluminate long-lasting phosphor. The method comprises the following steps: 1) taking the following raw materials according to the stoichiometric ratio: SrCO 3 、Al 2 o 3 、Eu 2 o 3 and Dy 2 o 3 , and weigh the flux H at the same time 3 BO 3 、BaF 2 ; 2) Grinding the raw materials weighed in step 1) and the flux to obtain a slurry; 3) suctioning the slurry; 4) drying the blank, and then sintering in a weak reducing atmosphere to obtain the europium , Dysprosium co-doped strontium aluminate long afterglow phosphor. In this method, a certain concentration of boric acid / barium fluoride is added as a combined flux when the phosphor powder is sintered by the high-temperature solid-state method, so that the luminous intensity of the sample powder sintered at a certain temperature is greatly improved, and the afterglow time is also extended. The afterglow performance of the phosphor is improved.
Owner:RENMIN UNIVERSITY OF CHINA
mg 2+ /ge 4+ replace ga 3+ doped cr 3+ Zinc gallate-based near-infrared long afterglow material and preparation method
ActiveCN110041928BGood dispersionImprove uniformityLuminescent compositionsBiological imagingZinc gallate
The invention belongs to the field of material science and discloses Mg 2+ / Ge 4+ replace Ga 3+ of Cr-doped 3+ Zinc gallate-based near-infrared long afterglow material and preparation method. Use the sol-gel method, add a certain complexing agent, and use Mg 2+ / Ge 4+ Ion pairs replace Ga 3+ Producing more traps, the near-infrared long afterglow nano-phosphor ZnGa is obtained by calcining at 700-1400°C. 2‑x (Mg 2+ / Ge 4+ ) x O 4 :yCr 3+ It has small size, good dispersion, excellent uniformity, good luminescence performance, long afterglow and other excellent properties, and can be well used in the field of biological imaging. The invention is simple to operate, low in cost, green and environmentally friendly, and provides a good theoretical basis for the preparation of new commercial phosphors, and has extremely high guiding significance and application prospects.
Owner:NORTHEASTERN UNIV LIAONING
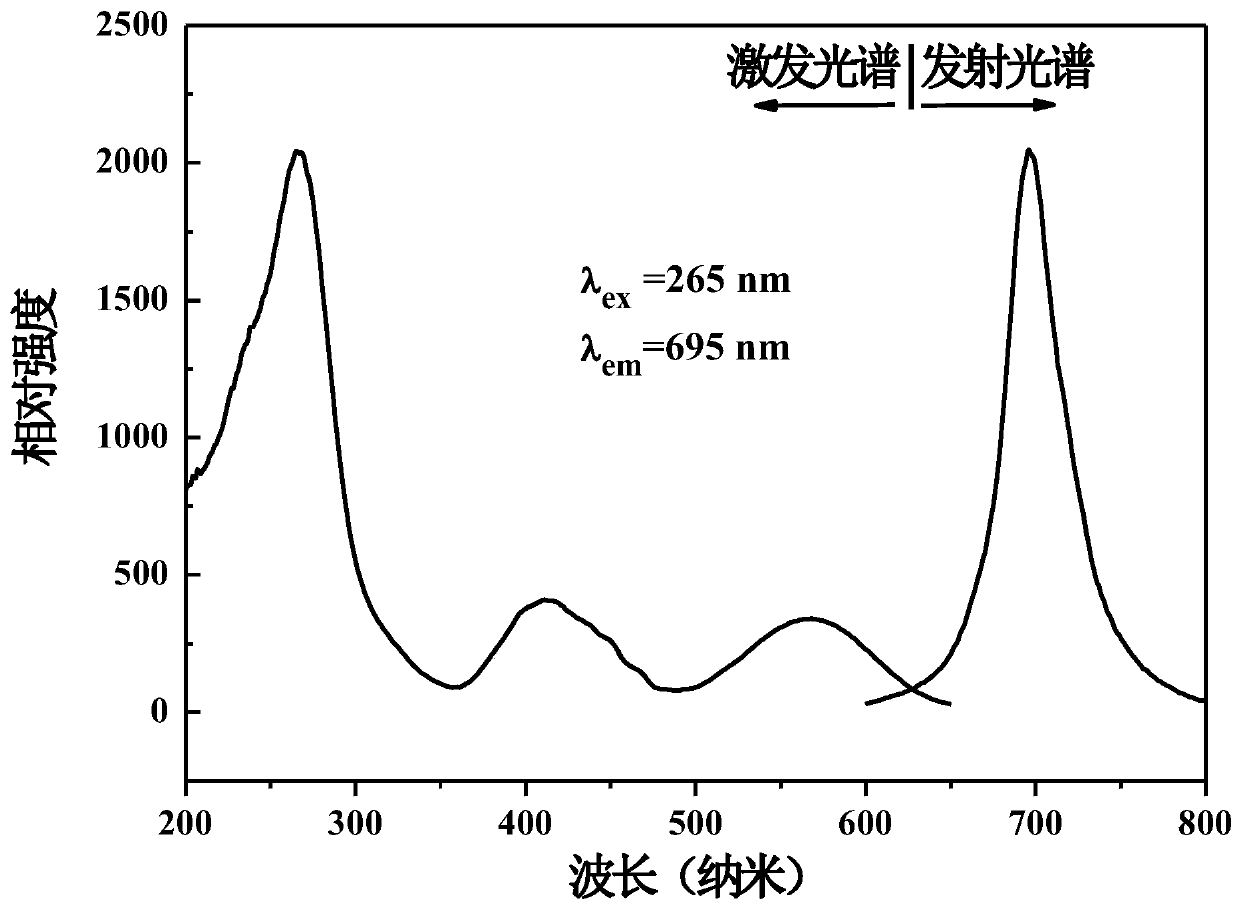
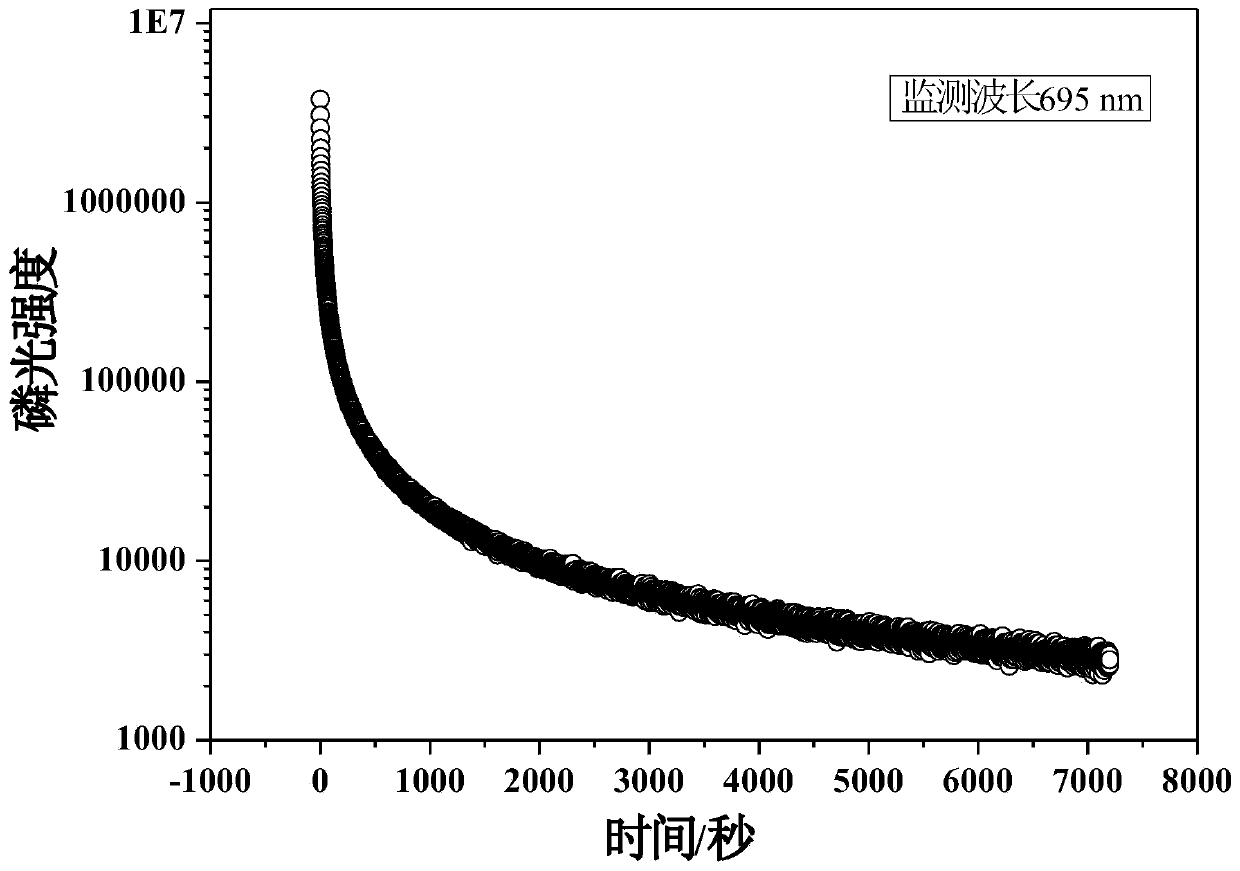
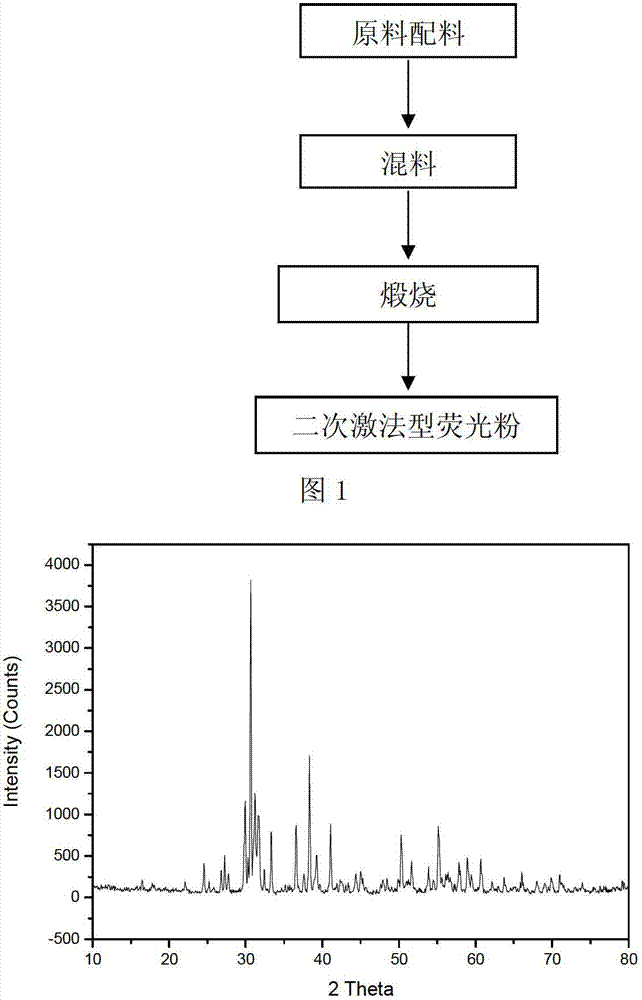
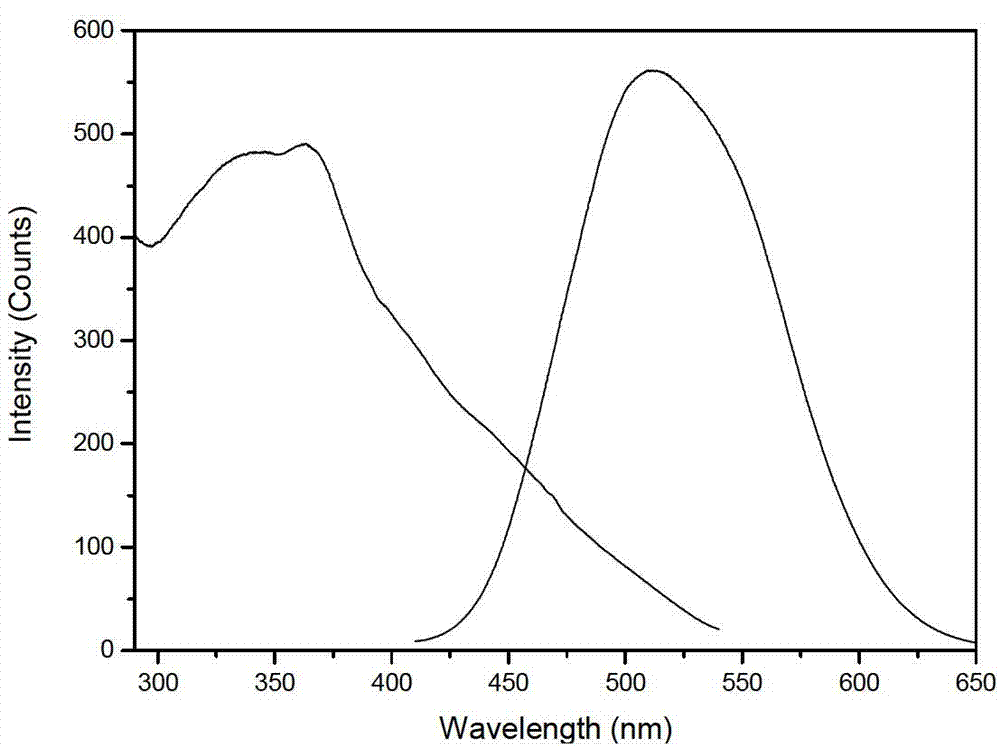
Secondarily-excited type silicon-aluminate long-afterglow fluorescent powder and preparation method thereof
Owner:重庆地恩科技开发有限责任公司
Preparation method of a nano-sized near-infrared light long afterglow material
InactiveCN108949174BHas a synthesis temperatureExcellent afterglow performanceLuminescent compositionsNanoparticleCentrifugation
The invention relates to a long-afterglow luminescent material, and particularly provides a preparing method of a nanometer near-infrared long-afterglow material. The method includes mixing Na2CO3, Ga2O3 and GeO2 according to a stoichiometric ratio; grinding and calcining the mixture to obtain Na2Ga<2(1-x-y)>Ge<x>O<4-x> powder; preparing a suspension from the powder; mixing the suspension with Zn(CH3COO)2 and Cr(CH3COO)3 in a stoichiometric ratio to perform ion exchange to obtain a precursor of the near-infrared long-afterglow material; and calcining the precursor, or transferring the precursor to a reaction kettle with a polytetrafluoroethylene liner, performing a hydrothermal reaction and centrifugation, then washing a product with water and ethanol alternatively, drying a product and calcining the product to obtain the nanometer near-infrared long-afterglow material. The method is simple, the calcining temperature is relatively low, and the prepared nanoparticles have good afterglowperformance and are prone to large-scale promotion and application.
Owner:XIAMEN UNIV
Preparation method and product of a rare earth aluminate-based composite red luminescent material
ActiveCN108822830BGood light colorImproved color stabilityLuminescent compositionsPhysical chemistryGreen-light
The invention discloses a preparation method and product of a rare-earth aluminate-based composite red luminescent material. The preparation method comprises the following steps: preparing a luminescent substrate-polydopamine compound, concretely mixing polydopamine particles, absolute ethyl alcohol and a rare-earth aluminate luminescent material, or mixing dopamine hydrochloride, a rare-earth aluminate luminescent material, absolute ethyl alcohol and tris(hydroxymethyl)aminomethane, and carrying out ultrasonic dispersing, heating, reacting, filtering, washing and drying; preparing an aluminate-based composite red luminescent material, concretely mixing the luminescent substrate-polydopamine compound, absolute ethyl alcohol, ethylene glycol and a light conversion agent; and grinding the luminescent material. According to the invention, excellent luminescent performance of the rare-earth aluminate and the characteristics of organic fluorescent powder are utilized, and the blue light-green light is converted to red light. The problem of difficult combination of an inorganic fluorescent material and an organic fluorescent material is solved by using polydopamine, and the performance of the red composite luminescent material is improved.
Owner:JIANGNAN UNIV
Method for preparing strontium aluminate long-persistence luminescent materials based on nanometer fusing assistants
InactiveCN101768439BLarge particle sizeFluorescence enhancementChemical industryLuminescent compositionsReaction temperaturePrinting ink
The invention provides a method for preparing strontium aluminate long-persistence luminescent materials based on nanometer fusing assistants. The invention has the technical scheme that the method comprises the following steps: simultaneously preparing a nanometer ammonium pentaborate powder fusing assistant A and a nanometer aluminium borate powder fusing assistant B by a liquid phase method; then, obtaining boron of a mixed fusing assistant and a defect forming assistant through the introduction of the fusing assistant A and the fusing assistant B; and finally, using solid-phase reaction for preparing the long-persistence luminescent materials at the low reaction temperature. The invention overcomes the defects of overhigh temperature required by the reaction, high cost of equipment used in the reaction, high consumption of energy required in the synthesis process, poor luminescent performance of products and the like when the existing solid-phase method is used for preparing the strontium aluminate long-persistence luminescent materials. At the same time, the invention also solves the problems that the traditional products only pursue the persistence time, so the fusing assistant with high boron content is added, the strontium aluminate reaction products can easily obtain the excessive sintering, and the application effect of the pulverized strontium aluminate reaction products is poor. The luminescent materials of the invention are mainly applied to the fields of safety passage display, luminescent printing ink, luminous lighting and light detection, and are also usedfor novel energy-saving LED lamps with persistence.
Owner:XIANGTAN UNIV
Blue light fluorescent powder for ultraviolet light excitation and preparation method thereof
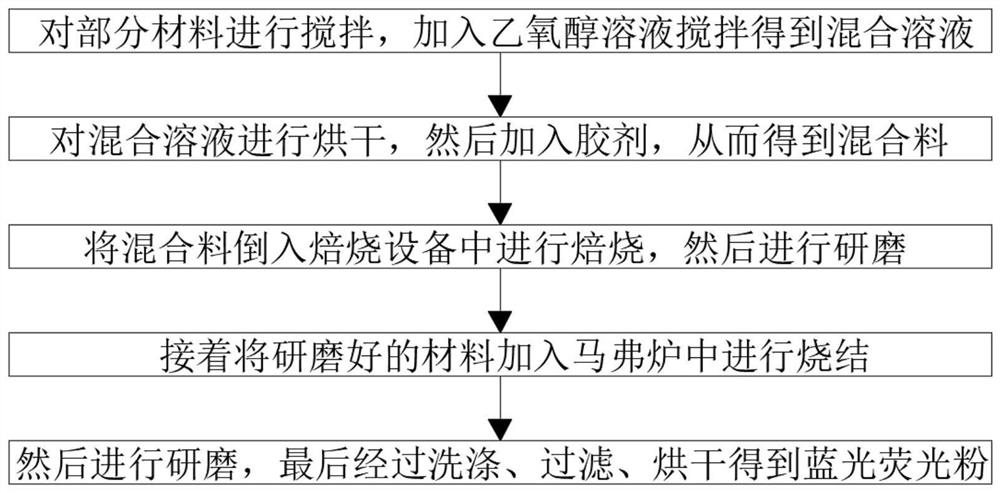
InactiveCN113980674AImprove performanceExcellent afterglow performanceLuminescent compositionsHexamethylenetetramineUltraviolet lights
The invention belongs to the field of anti-counterfeiting, and particularly relates to blue light fluorescent powder for ultraviolet excitation and a preparation method thereof. In allusion to the problems of unstable emission peak value and quantum efficiency and weak afterglow performance in the prior art, the invention proposes the following scheme: the blue light fluorescent powder is prepared from the following components in parts by weight: 60 parts of Ca3Ga4, 30 to 34 parts of a maleic anhydride-vinyl acetate linear alternating copolymer, 30 to 34 parts of nitrate, 20 to 24 parts of melamine, 16 to 20 parts of glue, 6 to 10 parts of urotropin and a proper amount of an ethoxy alcohol solution, the glue is maleic anhydride modified rosin glyceride mixed glue, the performance of the fluorescent powder can be enhanced through the Ca3Ga4, the maleic anhydride-vinyl acetate linear alternating copolymer, the nitrate, the melamine, the glue, the urotropine and a proper amount of the ethoxy alcohol solution, the emission peak value and the quantum efficiency are properly matched, the requirements of the commercial anti-counterfeiting fluorescent powder are met, and the afterglow performance of the fluorescent powder is further improved.
Owner:KUNMING UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com