Composite solid super acidic catalyst, preparation method thereof and method for catalytically synthesizing N,N-diethylaniline by using solid super acidic catalyst
A technology of solid superacid and diethylaniline, applied in the direction of catalyst activation/preparation, preparation of amino compounds, chemical instruments and methods, etc., can solve the problems of small number of acidic sites, long equilibration time, short service life, etc., to achieve High selectivity, simple preparation method, and the effect of improving the service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
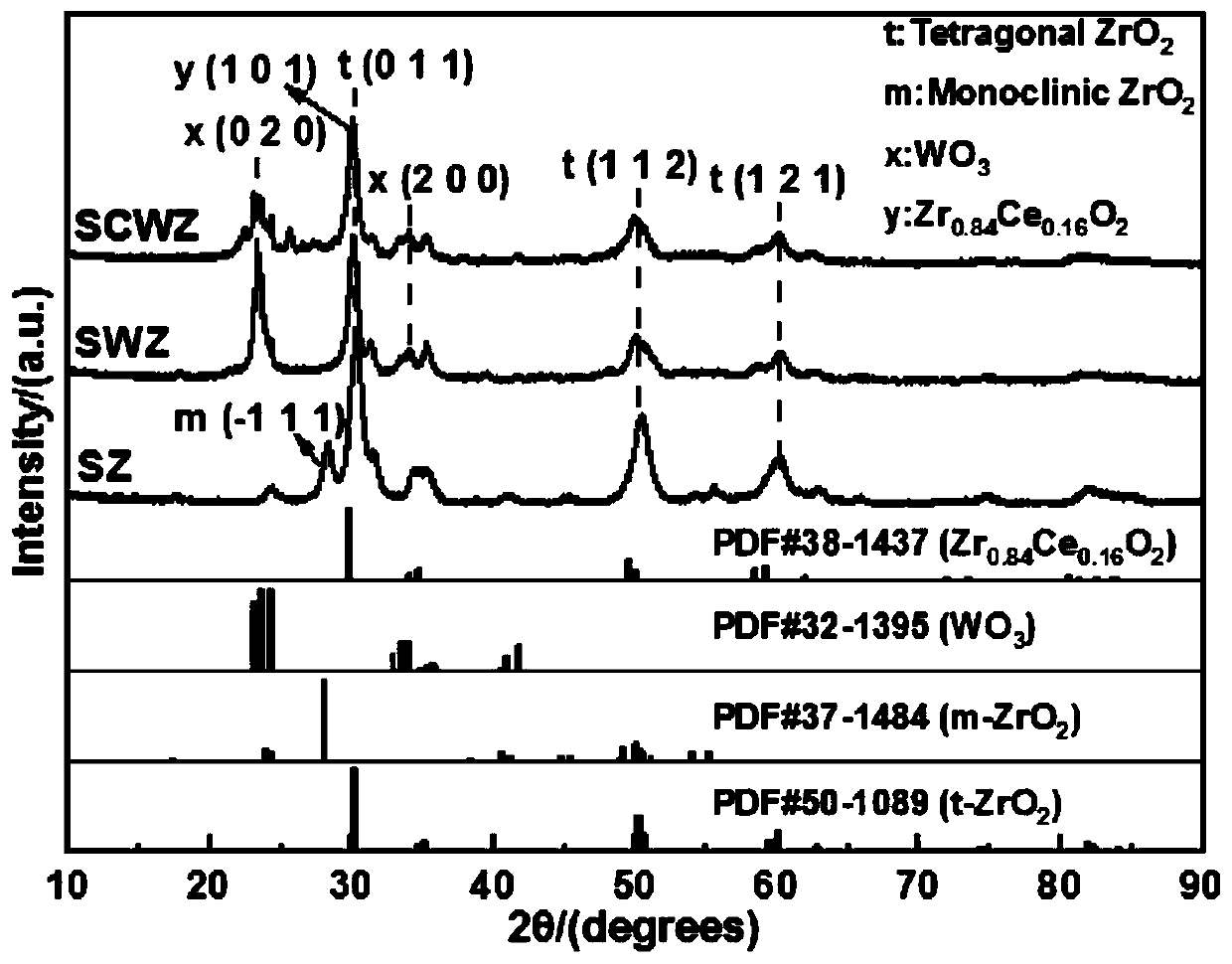
[0026] The composite solid superacid catalyst of this embodiment, catalyzer is made of composite carrier CeO 2 -WO 3 -ZrO 2 and surface-loaded SO 4 2- composition. Wherein, the molar ratio in the carrier n(W):n(Zr)=0.5:1, n(Ce):n(Zr)=0.1:1.
[0027] The preparation method of this catalyst comprises the steps:
[0028] (1) Preparation of CeO 2 -WO 3 -ZrO 2 Carrier precursor: ZrOCl 2·8H 2 O, (NH 4 ) 6 h 2 W 12 o 40 ·xH 2 O and Ce(NO 3 ) 3 ·6H 2 O was placed in a three-necked flask according to the above molar ratio, and water was added to dissolve it completely, and concentrated NH was added dropwise with a constant pressure dropping funnel 3 ·H 2 O (25wt%), and vigorously stirred until the pH = 9 ~ 11; continued to stir for 10 ~ 15h, ultrasonic 3-5h, and then transferred to a polytetrafluoroethylene-lined stainless steel hydrothermal synthesis kettle, at 120 ° C Aging for 22 hours; after cooling, filter with suction, dry the precipitate at 120°C for 22 hour...
Embodiment 2
[0044] The single dimension experiment of the mol ratio of embodiment 2 W and Zr
[0045] The basic steps are the same as in Example 1, except that the ratio of W and Zr in the carrier is used, and N,N-diethylaniline is catalyzed by a catalyst, and the obtained results are shown in Table 2 below.
[0046] The conversion rate of the aniline of table 2 embodiment 2 and the selectivity result of N,N-diethylaniline
[0047] serial number no W :n Zr
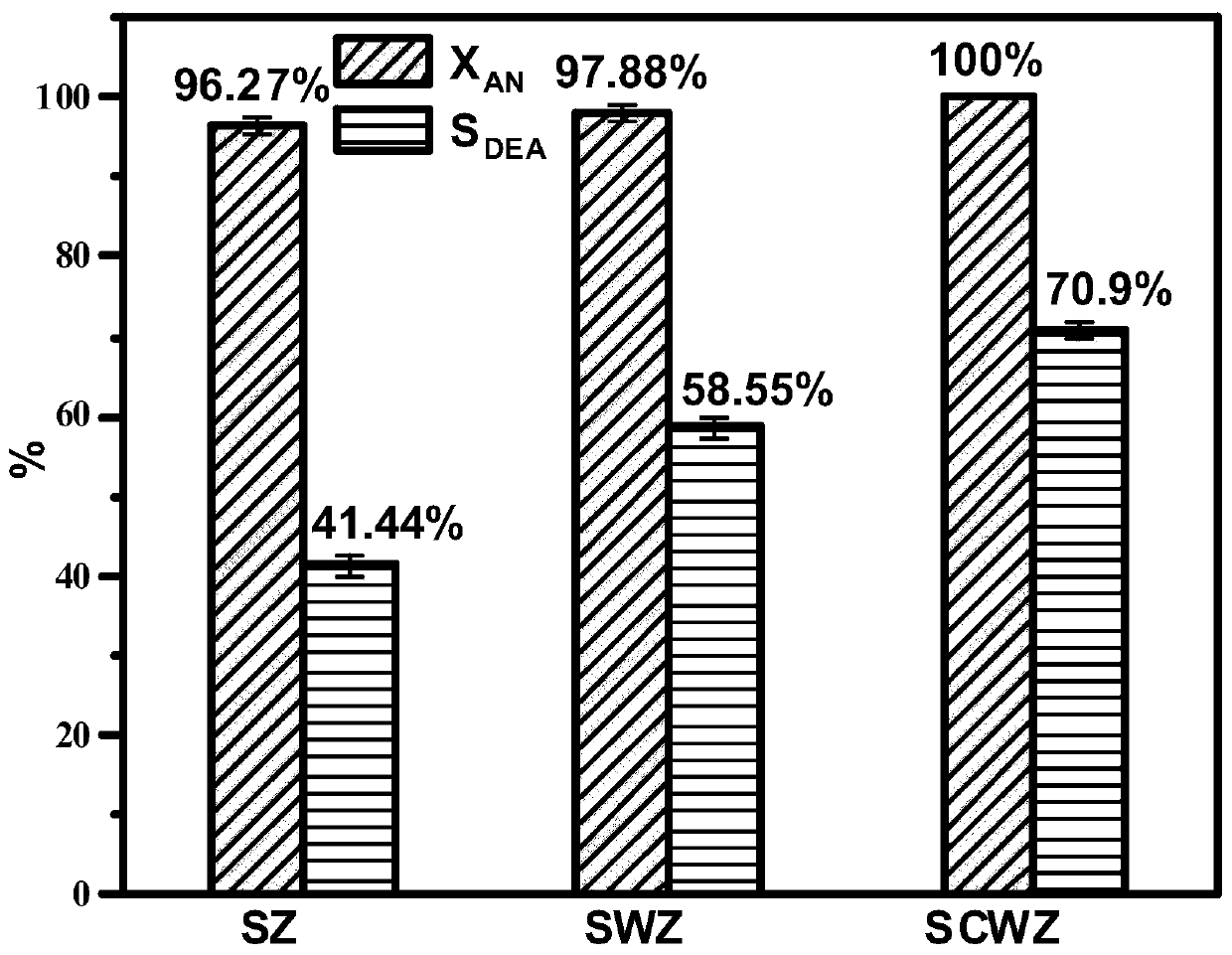
[0048] It can be seen from Table 2 that the present invention uses a catalyst with a molar ratio of n(W):n(Zr)=0.1~0.9:1, which can make the conversion rate of aniline reach 95.41~100%, and N,N-diethyl The selectivity of aniline can reach 53.84~70.9%.
Embodiment 3
[0049] The single dimension experiment of the molar ratio of embodiment 3 Ce and Zr
[0050] The basic steps are the same as in Example 1, except that the molar ratio of Ce and Zr is used, and the prepared catalyst is used to prepare N,N-diethylaniline, and the obtained results are shown in Table 3 below.
[0051] The conversion rate of the aniline of table 3 embodiment 3 and the selectivity result of N,N-diethylaniline
[0052] serial number no Ce :n Zr
[0053] It can be seen from Table 3 that the present invention uses a catalyst with a molar ratio n(Ce):n(Zr)=0.05~0.15:1, which can make the conversion rate of aniline reach 98.08~100%, and N,N-diethylaniline The selectivity can reach 56.34~70.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com