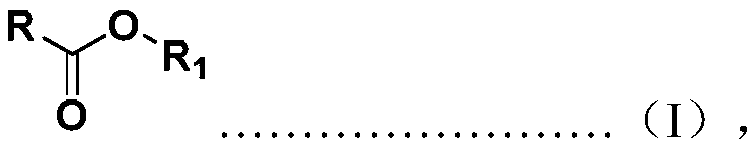
Borohydride reduction stabilizing system and method for reducing ester into alcohol
A borohydride and stable system technology, applied in chemical instruments and methods, preparation of organic compounds, preparation of hydroxyl compounds, etc., can solve problems such as easy decomposition, reduce hydrogen generation, reduce excessive use, and reduce process risks Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066]
[0067] After dissolving methyl p-nitrobenzoate (110g, 0.61mol) in methanol (440mL, 4V) and stirring, pump A into the coil at a speed of 3.16g / min, sodium borohydride (33.86g, 0.92 mol), MeONa (0.98g, 0.018mol) was dissolved in methanol (110mL, 1V) and pumped to In the coil tube, the coil tube was immersed in an oil bath at 40°C, the retention time was 60min, the outlet was sampled by HPLC, and the outflow system was put into a 1L four-neck flask for quenching and extraction, and then vacuum distillation gave 79.3g of yellow liquid fraction product, with a yield of 85% %.
Embodiment 2
[0069]
[0070] Methyl p-nitrobenzoate (110g, 0.61mol) was dissolved in methanol (440mL, 4V) and stirred, then pumped into the coil with pump A at a speed of 4.74g / min, sodium borohydride (33.86g, 0.92 mol), MeONa (0.98g, 0.018mol) were dissolved in methanol (110mL, 1V) and pumped to 240mL with pump B at a speed of 1.26g / min In the coil, the coil is immersed in an oil bath at 50°C, the retention time is 40min, the outlet is sampled by HPLC, and the outflow system enters a 1L four-neck flask for quenching and extraction, and then vacuum distillation to obtain 84g of yellow liquid fraction product, with a yield of 90.3% .
Embodiment 3
[0072]
[0073] Methyl p-nitrobenzoate (110g, 0.61mol) was dissolved in methanol (440mL, 4V) and stirred, then pumped into the coil with pump A at a speed of 9.48g / min, sodium borohydride (33.86g, 0.92 mol), MeONa (0.98g, 0.018mol) were dissolved in methanol (110mL, 1V) and pumped to 240mL with pump B at a speed of 2.52g / min In the coil tube, the coil tube was submerged in a 60°C oil bath, the retention time was 20min, the outlet was sampled by HPLC, and the outflow system was put into a 1L four-necked flask for quenching and extraction, and then vacuum distillation to obtain 88.6g of yellow liquid fraction product, with a yield of 95% %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com