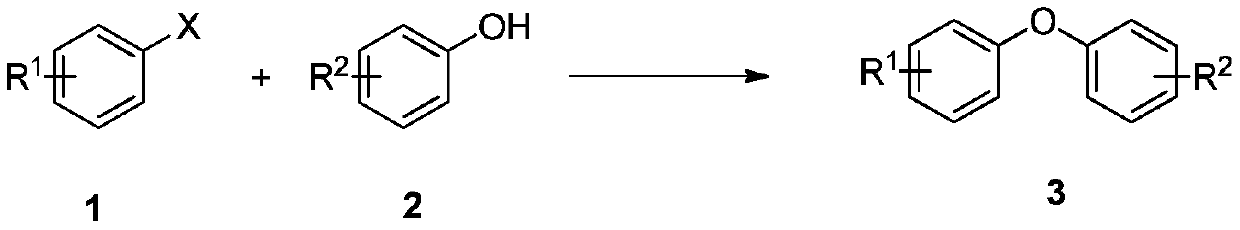
Method for preparing diaryl ether compound
A technology for diaryl ethers and compounds is applied in the field of novel preparation of diaryl ether compounds, can solve problems such as high cost, and achieve the effects of simple operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
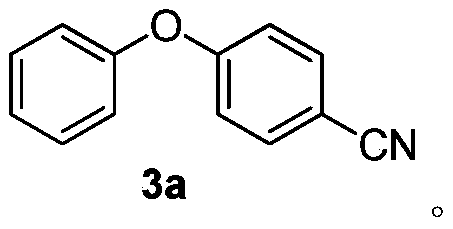
[0024] A method for preparing 4-cyano diaryl ether (4-phenoxybenzonitrile) 3a, comprising the following experimental steps:
[0025] In the reactor, add compound 4-cyanoiodobenzene 1a (0.23g, 1.0mmol, 1.0eqv.), DMSO (3ml), Cs 2 CO 3 (0.65g, 2.0mmol, 2.0eqv.), phenol 2a (0.113g, 1.2mmol, 1.2eqv.), stirred at room temperature for 40 minutes. Then put it in an oil bath at 80°C and react under light for 10 h. After the reaction was detected by TLC, the reaction liquid was filtered, extracted and column chromatographed to obtain the target product 3a with a yield of 79%.
[0026]
[0027] 1 H NMR (CDCl 3 ,400MHz,)δ(ppm)7.60(d,J=8.0Hz,2H,Ar-H),7.44-7.40(m,2H,Ar-H),7.26-7.23(m,1H,Ar-H), 7.08(m,2H,Ar-H),7.00(m,2H,Ar-H);
[0028] 13 C NMR (101MHz, CDCl 3 )δ161.71, 154.81, 139.29, 134.29, 134.18, 130.29,
[0029] 125.21, 122.46, 120.47, 118.92, 118.19, 117.93, 105.80.
Embodiment 2
[0031] In the reactor, add compound 4-cyanoiodobenzene 1a (2.3g, 10mmol, 1.0eqv.), DMSO (30ml), Cs 2 CO 3 (6.5g, 20mmol, 2.0eqv.), phenol 2a (1.13g, 12mmol, 1.2eqv.), stirred at room temperature for 40 minutes. Then put it in an oil bath at 80°C and react under light for 10 h. After the reaction was detected by TLC, the reaction solution was filtered, extracted and column chromatographed to obtain the target product 3a with a yield of 76%.
Embodiment 3
[0033] In the reactor, add compound 4-cyanoiodobenzene 1a (0.023g, 0.10mmol, 1.0eqv.), DMSO (0.3ml), Cs 2 CO 3 (0.065g, 0.20mmol, 2.0eqv.), phenol 2a (0.0113g, 0.12mmol, 1.2eqv.), stirred at room temperature for 40 minutes. Then put it in an oil bath at 80°C and react under light for 10 hours. After the reaction was detected by TLC, the reaction solution was filtered, extracted and column chromatographed to obtain the target product 3a with a yield of 83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com