Method for synthesizing optically pure (R)-3-carbamoymethyl-5-methylhexanoic acid
A technology of carbamoylmethyl and methylhexanoic acid, which is applied in the field of synthesis of optically pure 3-carbamoylmethyl-5-methylhexanoic acid, can solve the problem of high toxicity of resolving agents, high raw material prices, Slow reaction speed and other problems, to achieve the effect of shortening the reaction time, low raw material cost and fast reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
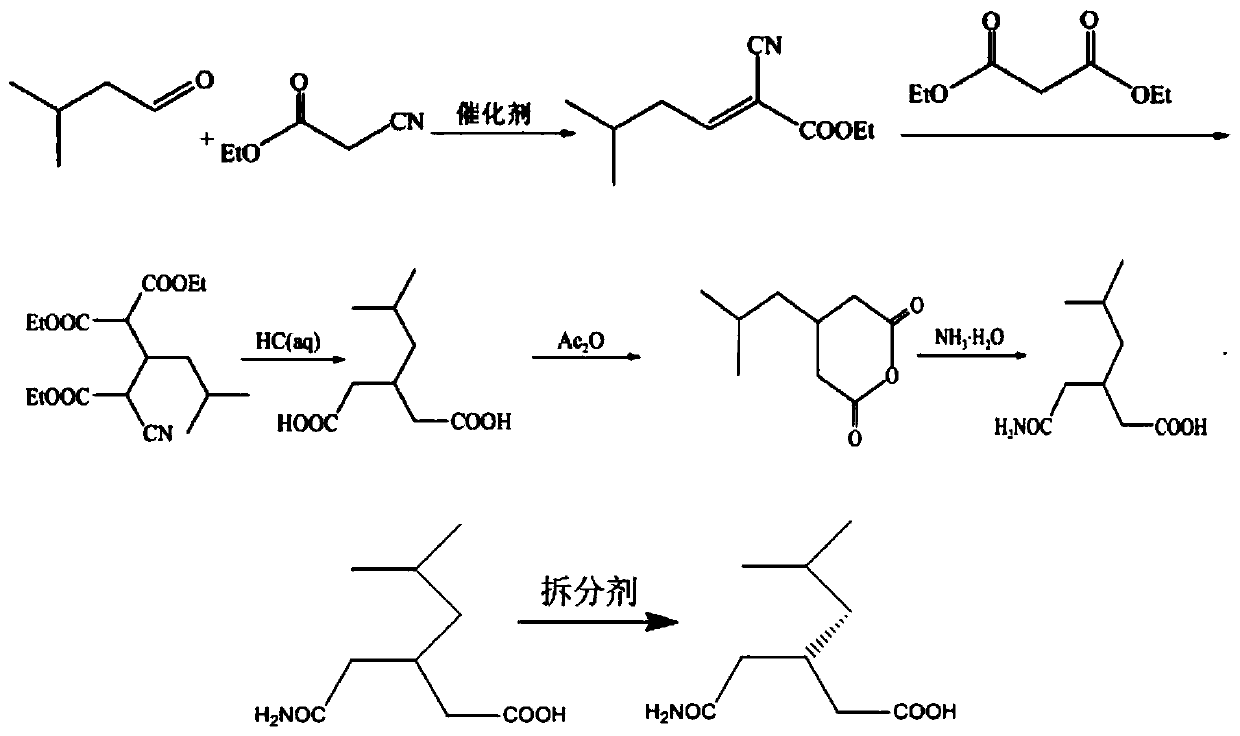
[0022] A kind of synthetic method of optically pure (R)-3-carbamoylmethyl-5-methylhexanoic acid of the present invention comprises the following steps:
[0023] Step 1: Add 56.5g (0.5mol) of ethyl cyanoacetate and 100ml of n-hexane into a 500ml reaction flask, stir evenly and control the temperature range at 0-5°C, then slowly add 0.5g of triethylenediamine and iso 43g (0.5mol) of valeraldehyde, the color of the solution gradually becomes light yellow, stir at room temperature, and then heat up to 90-100°C, use a water separator to reflux and separate water, react until no water comes out, cool down; concentrate under reduced pressure, Obtain ethyl 2-cyano-5-methyl-2-ene hexanoate;
[0024] Step 2, directly add 72.2g (0.5mol) of diethyl malonate and 5g of triethylenediamine to the product obtained in step 1, stir and react for 10 hours, directly add 500ml of hydrochloric acid, heat to reflux until the reaction is completed, and cool to room temperature Extract and concentrate...
Embodiment 2
[0029] Step 1: Add 56.5g (0.5mol) of ethyl cyanoacetate and 100ml of n-hexane into a 500ml reaction flask, stir evenly and control the temperature range at 0-5°C, then slowly add 0.5g of triethylenediamine and iso 43g (0.5mol) of valeraldehyde, the color of the solution gradually becomes light yellow, stir at room temperature, and then heat up to 90-100°C, use a water separator to reflux and separate water, react until no water comes out, cool down; concentrate under reduced pressure, Obtain ethyl 2-cyano-5-methyl-2-ene hexanoate;
[0030] Step 2, directly add 72.2g (0.5mol) of diethyl malonate and 5g of triethylenediamine to the product obtained in step 1, stir and react for 10 hours, directly add 500ml of hydrochloric acid, heat to reflux until the reaction is completed, and cool to room temperature Extracted twice with dichloromethane, concentrated under reduced pressure to obtain 3-isobutyl-2-cyano-4-ethoxycarbonyl-glutaric acid ethyl ester;
[0031] Combine the extracted...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com