Application of antibacterial peptide Lchamp2-3 in anti-tumor cell proliferation medicine
A technology for anti-tumor cells and tumor cells, applied in the application field of antibacterial peptide Lchamp2-3 in anti-tumor proliferation drugs, which can solve the problems of limited curative effect data
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The antimicrobial peptide Lchamp2-3 was found in large yellow croaker in vivo, and the corresponding amino acid sequence of its mature peptide was: GSPARCRFCCRCCPGMRGCGICCRF. For the specific method of obtaining it, please refer to the literature: Mu Yinnan, Huo Jieying, GuanYanyun, Fan Dingding, Xiao Xiaoqiang, Wei Jingguang, Li Qiuhua, Mu Pengfei, AoJingqun, Chen Xinhua. An improved genome assembly for Larimichthys croceareveals hepcidin gene expansion with diversified regulation .[J].Communications biology,2018,1.
[0024] Dissolve 2 mg of Lchamp2-3 lyophilized powder in 1 mL of PBS, and use it as Lchamp2-3 mother solution for later use.
Embodiment 2
[0026] Cytotoxicity Test:
[0027] Dissolve 2.5 g of MTT powder in 50 mL of PBS solution to prepare a 10-fold concentration of MTT mother liquor with a concentration of 50 mg / mL, and filter to sterilize. Take out the mother solution when used, and dilute ten times with the corresponding serum-free and phenol red-free medium to become the MTT working solution.
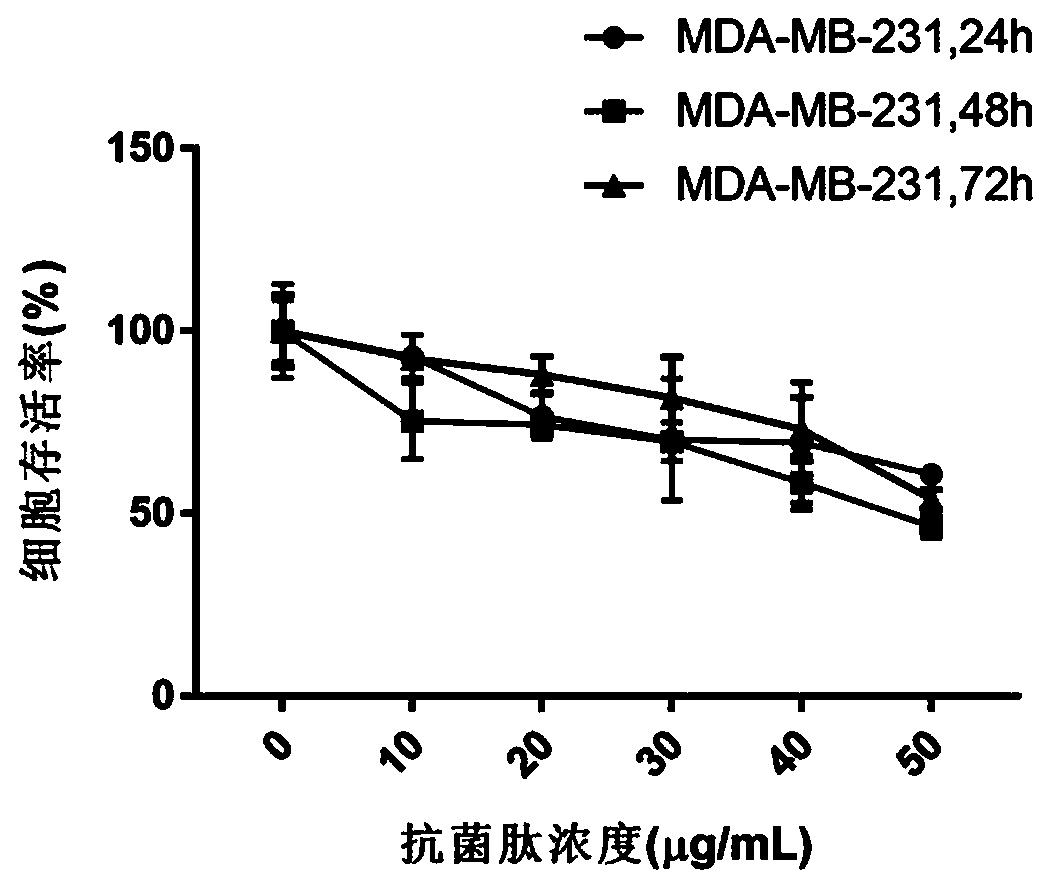
[0028] MDA-MB-231 cells were digested and blown into a uniform cell suspension, and the cell concentration in the suspension was calculated with a cell counting plate, and diluted to 80,000 cells per milliliter, and then spread into a 96-well plate to control the number of cells per well. Into 100μL cell suspension, 8000 cells. After incubating overnight at 37°C, add Lchamp2-3 mother solution, dilute to final concentrations of 0, 10, 20, 30, 40, and 50 μg / mL, and set 5 wells for each concentration to repeat. Put them back into a constant temperature incubator at 37°C for cultivation. After 24, 48, and 72 hours of drug...
Embodiment 3
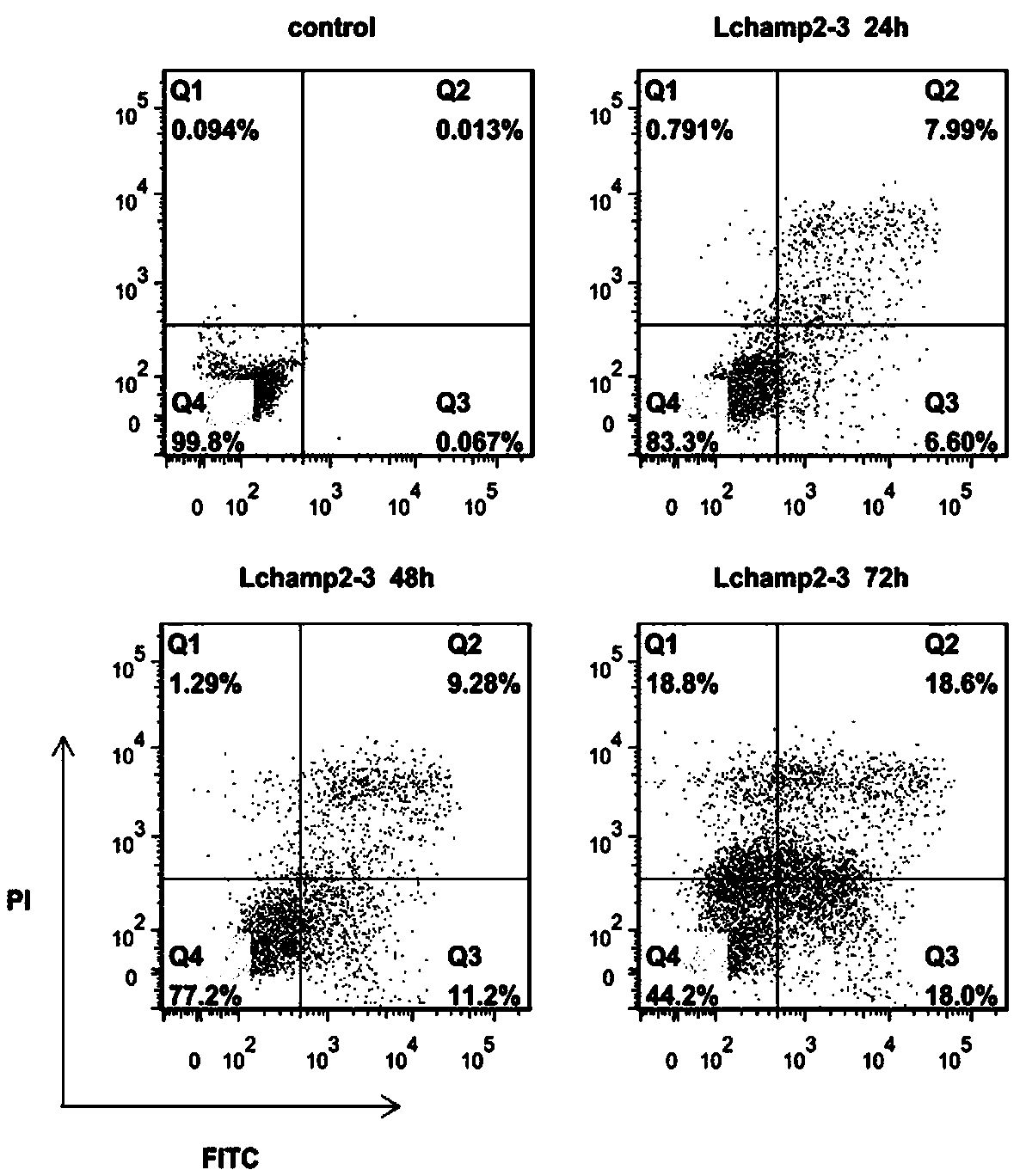
[0034] Lchamp2-3 induced cell apoptosis, MDA-MB-231 cells in good growth state were taken, digested with trypsin and counted. According to 1.5×10 5 cell / well Cells were seeded into 6-well plates and cultured overnight in an incubator.
[0035] After the cells were completely adhered to the wall, the old medium was discarded and washed 3 times with PBS. Add L-15 complete medium with Lchamp2-3 content of 25 μg / mL to each well, incubate for 24, 48, and 72 hours respectively, discard the drug-containing medium, wash with PBS three times, digest and collect cells, and centrifuge at 1800 rpm for 5 min , discard the supernatant, and collect the cell pellet at the bottom of the centrifuge tube. Set up two single positive tubes, add 5 μL Annexin V-FITC and 10 μL PI respectively, add binding buffer buffer to a total volume of 500 μL in each tube; set negative control tubes, add 500 μL binding buffer buffer; experimental group and blank The control group needs to add 5 μL Annexin V-FI...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com