Transaminase mutant and its application
A technology of mutant and transaminase, applied in transaminase mutant and its application field, can solve the problems of loss of activity, poor water solubility, variability of wild transaminase and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
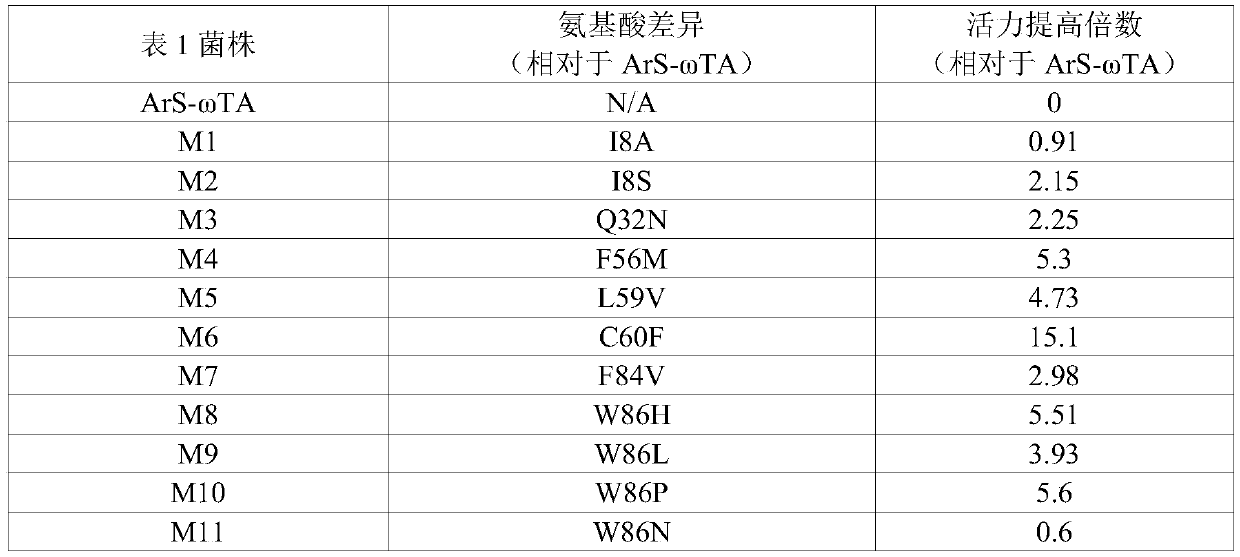
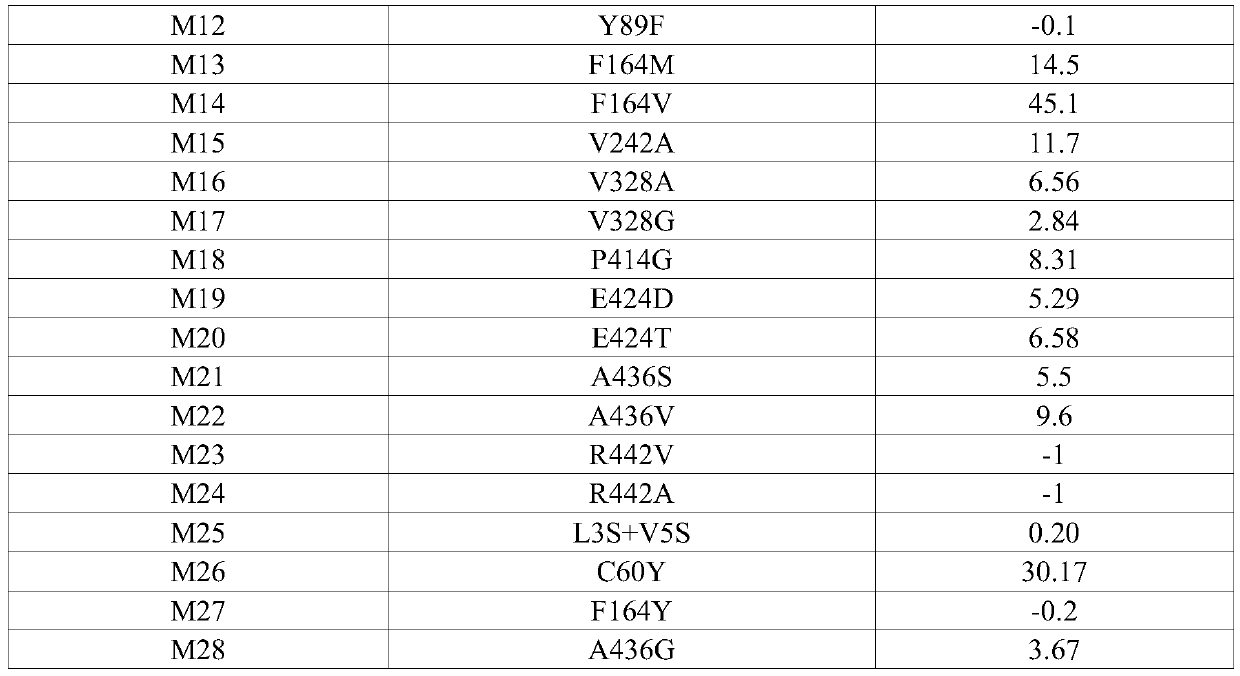
[0095] According to a typical embodiment of the present invention, the mutation further includes at least one of the following mutation sites or combinations of mutation sites: 7th, 32nd, 96th, 164th, 171st, 186th, 252nd, 384th, 389th, 391st, 394th, 404th, 411th, 420th, 423rd, 424th, 442nd, 452nd and 456th Preferably, the mutation further includes at least one of the following mutation sites: K7N, Q32L, K96R, V164L, E171D, S186G, V252I, Y384F, I389M, I389F, D391E, N394D, L404Q, L404Q, G411D, Q420R, Q420K, M423K, E424R, E424K, E424Q, R442H, R442L, G452S and K456R. Mutations are carried out by site-directed mutagenesis to change its amino acid sequence to achieve changes in protein structure and function, and then through directional screening methods to obtain transaminases with the above mutation sites, so these transaminase mutants have good resistance to organic solvents tolerance and high pH tolerance, and has high soluble expression characteristics and high activity chara...
Embodiment 1
[0116] Catalytic activity of ArS-ωTA mutant and wild enzyme on substrate 1 in organic solvent-free system:
[0117]
[0118] In a 10mL reaction bottle, weigh 100mg of the raw material, add 1mg of pyridoxal 5'-phosphate, add 2mM isopropylamine hydrochloride, add 250μL of crude enzyme solution of ArS-ωTA mutant or wild enzyme (0.05g of mutant wet cells are subjected to sonication Broken to obtain 20% crude enzyme solution (pH=8.5), add 0.41mL of 100mM PB8.5 to make the final volume of the system to 1mL, stir at 30°C for 16h, centrifuge the system at 12000rpm for 5min, take a sample of 200μL and add 2mL of acetonitrile to dissolve it. After being centrifuged at 12000rpm for 5min, the product conversion rate was detected by HPLC. The mutant information and results are shown in Table 9.
[0119] Table 9
[0120]
[0121] The results in Table 9 showed that the catalytic activity of the ArS-ωTA mutant to substrate 1 was greatly improved compared with the wild strain. After th...
Embodiment 2
[0123] Catalytic activity of ArS-ωTA wild enzyme and mutants to substrate 1 in organic solvent system (40%) DMSO:
[0124] In a 10mL reaction bottle, weigh 100mg of raw material (same as Example 1), add 1mg 5'-pyridoxal phosphate, add 2mM isopropylamine hydrochloride, add 250μL ArS-ωTA mutant or wild enzyme crude enzyme solution (0.05g The mutant wet cells were sonicated to obtain 20% crude enzyme solution (pH = 8.5), add 100mM PB8.5 0.01mL, add dimethyl sulfoxide 0.4mL to make the final volume of the system 1mL, and stir at 35°C for 16h, After the system was centrifuged at 12000rpm for 5min, 200 μL of the sample was added to 2mL of acetonitrile to dissolve, centrifuged at 12000rpm for 5min, and sent to HPLC to detect the conversion rate of the product. See Table 10 for mutant information and results.
[0125] Table 10
[0126]
[0127] ND: No Product Generation Detected
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com