Preparation method for cyclized intermediate of florfenicol
A florfenicol and intermediate technology, which is applied in the field of preparation of florfenicol cyclic compound intermediates, can solve the problems of difficult recovery of glycerol, low product yield, long reaction time and the like, and achieves strong reduction activity and selectivity. The effect of high sex and fast reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
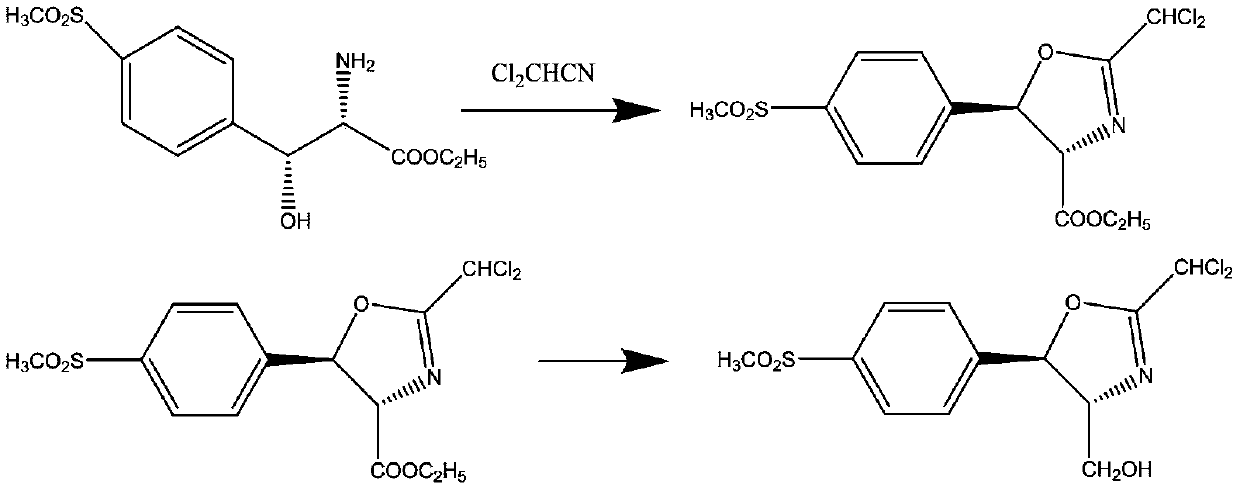
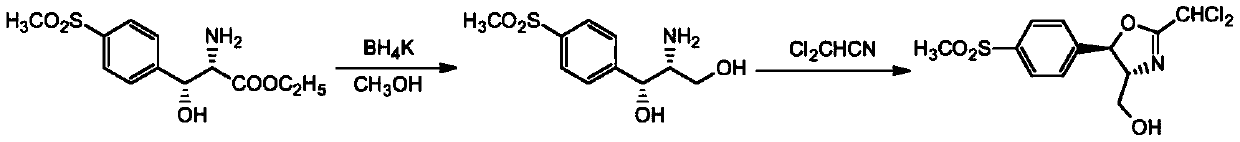
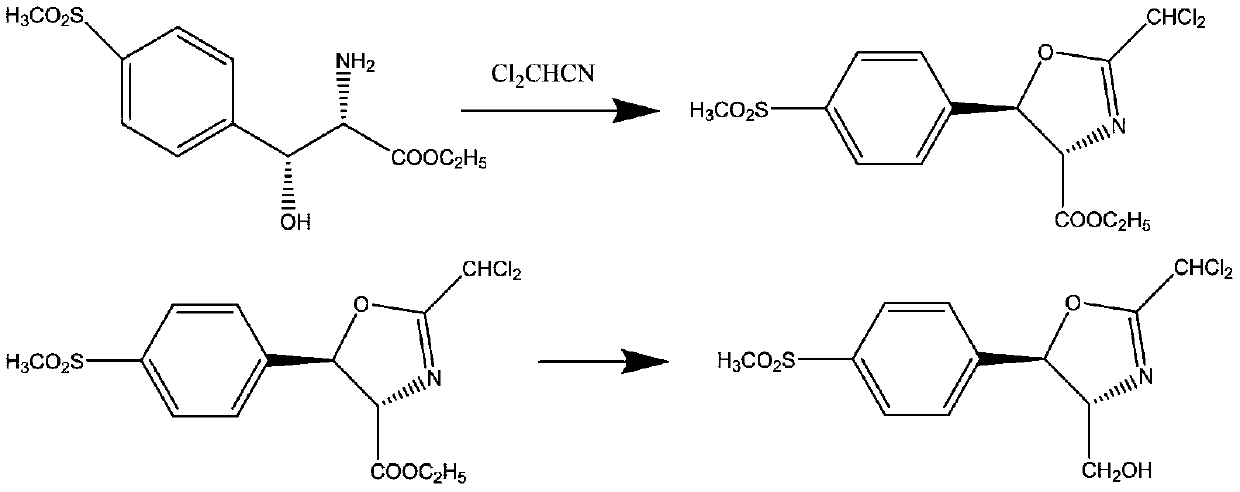
[0031] (1) Cyclization reaction: 287.3 g of D-threo-p-thymphenyl phenylserine ethyl ester was added to 2000 ml of ethanol, and then 132 g of dichloroacetonitrile was added. At a reaction temperature of 80 degrees, the reaction time was 2 hours. Obtain the cyclization product intermediate;
[0032] (2) Reduction reaction: the cyclization product obtained in step (1) does not need to be separated. After cooling down, 37.9 g of reducing agent sodium borohydride is added, and the reaction is carried out at 20 degrees for 4 hours. After the reaction is over, add water to lower the temperature and precipitate the product. After filtration, the key intermediate compound D-threo-2-(dichloromethyl)-4,5-dihydro-5-[p-(methylsulfonyl) is obtained after filtration. ) phenyl]-4-oxazolemethanol.
[0033] The product quality after drying is 320g, the yield is 94.6%, and the content is greater than 99%.
Embodiment 2
[0035] (1) Cyclization reaction: 287.3 g of D-threo-p-thymphenyl phenylserine ethyl ester was added to 2000 ml of methanol, and then 220 g of dichloroacetonitrile was added. At a reaction temperature of 40 degrees, the reaction time was 5 hours. Obtain the cyclization product intermediate;
[0036] (2) Reduction reaction: the cyclization product obtained in step (1) does not need to be separated. After cooling down, 107.9 g of reducing reagent potassium borohydride is added, and the reaction is carried out at 60 degrees for 2 hours. After the reaction is over, add water to lower the temperature and precipitate the product. After filtration, the key intermediate compound D-threo-2-(dichloromethyl)-4,5-dihydro-5-[p-(methylsulfonyl) is obtained after filtration. ) phenyl]-4-oxazolemethanol.
[0037] The product quality after drying is 330g, the yield is 97.6%, and the content is greater than 99%.
Embodiment 3
[0039] (1) Cyclization reaction: 287.3 g of D-threo-p-thymphenyl phenylserine ethyl ester was added to 2000 ml of methanol, and then 165 g of dichloroacetonitrile was added. At a reaction temperature of 60 degrees, the reaction time was 4 hours. Obtain the cyclization product intermediate;
[0040] (2) Reduction reaction: the cyclization product obtained in step (1) does not need to be separated, after cooling down, add 45 g of reducing agent lithium aluminum hydride, and react at 40 degrees for 3 hours. After the reaction is over, add water to lower the temperature and precipitate the product. After filtration, the key intermediate compound D-threo-2-(dichloromethyl)-4,5-dihydro-5-[p-(methylsulfonyl) is obtained after filtration. ) phenyl]-4-oxazolemethanol.
[0041] The product quality after drying is 333g, the yield is 98.5%, and the content is greater than 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com