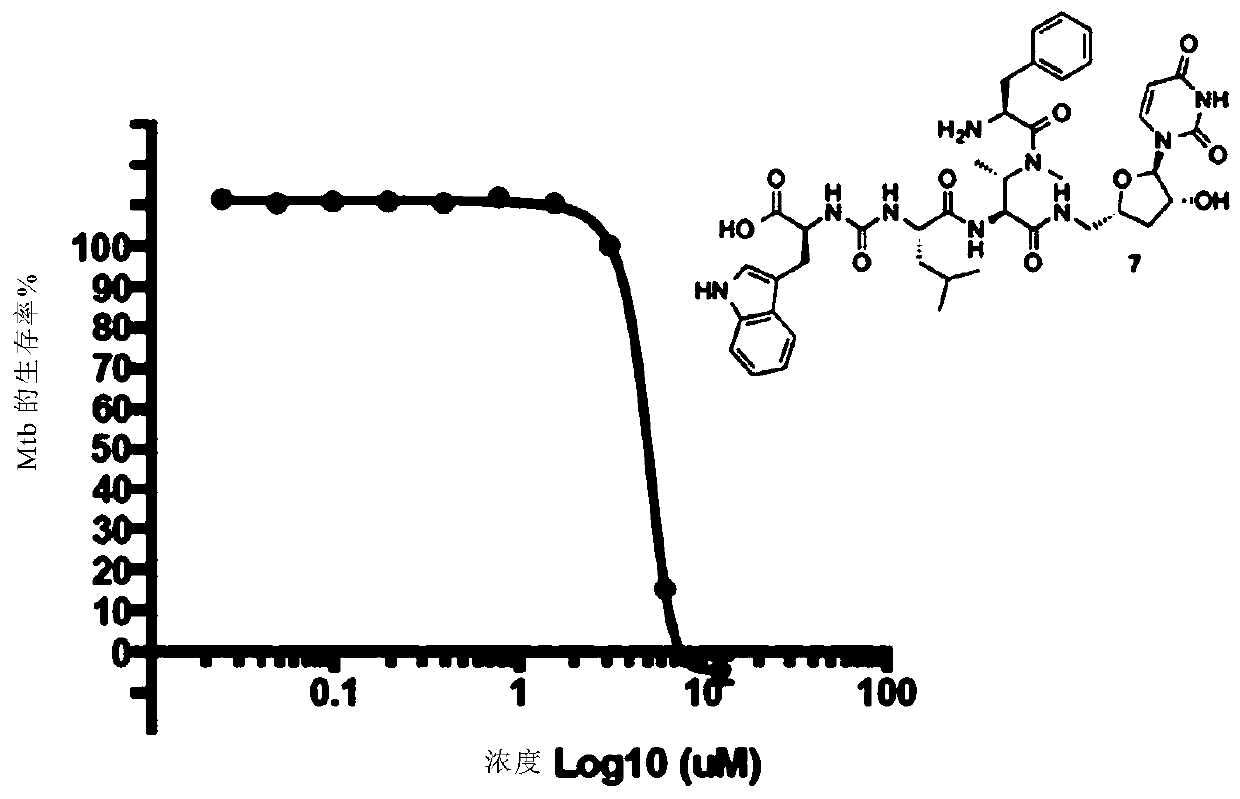
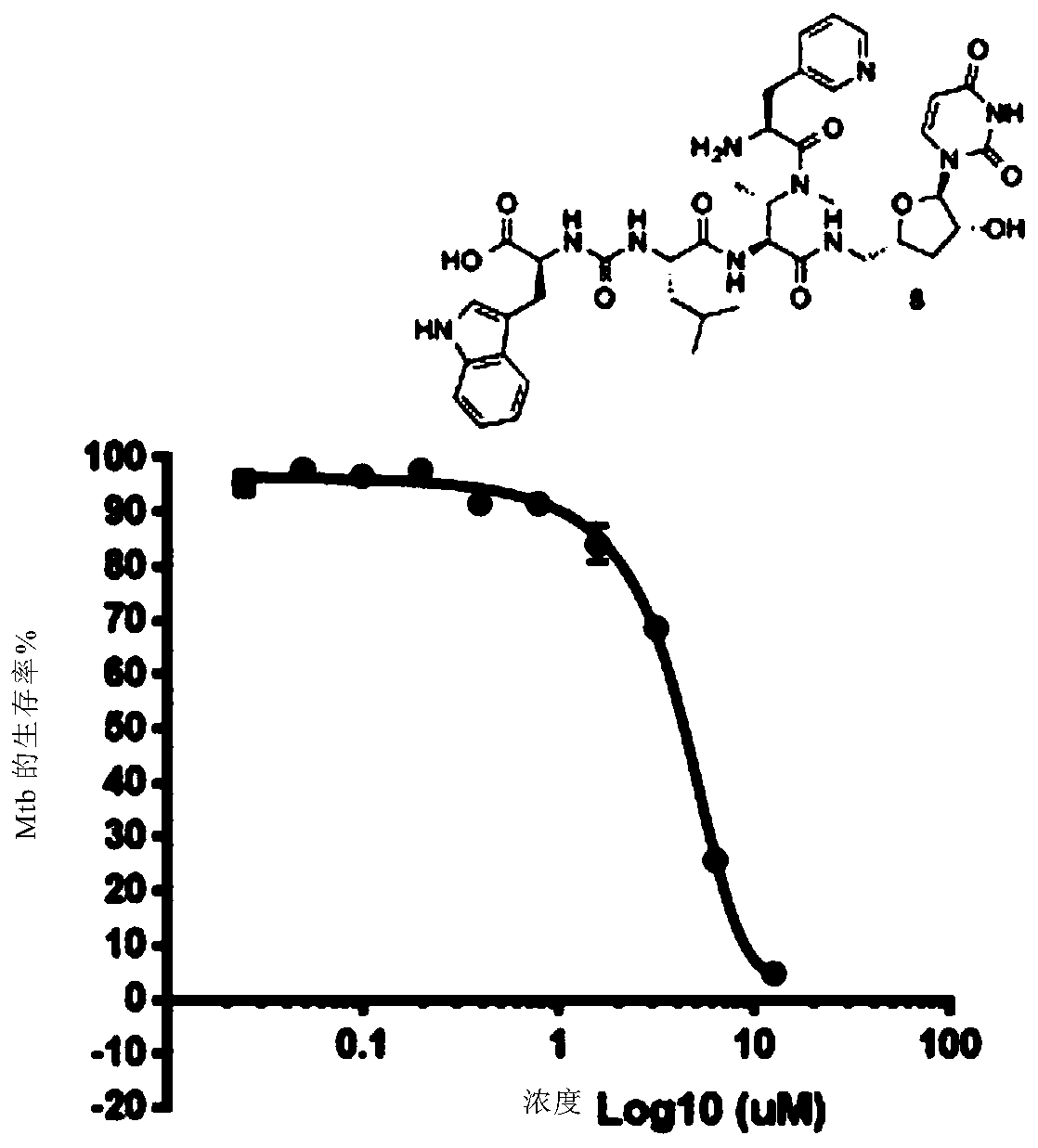
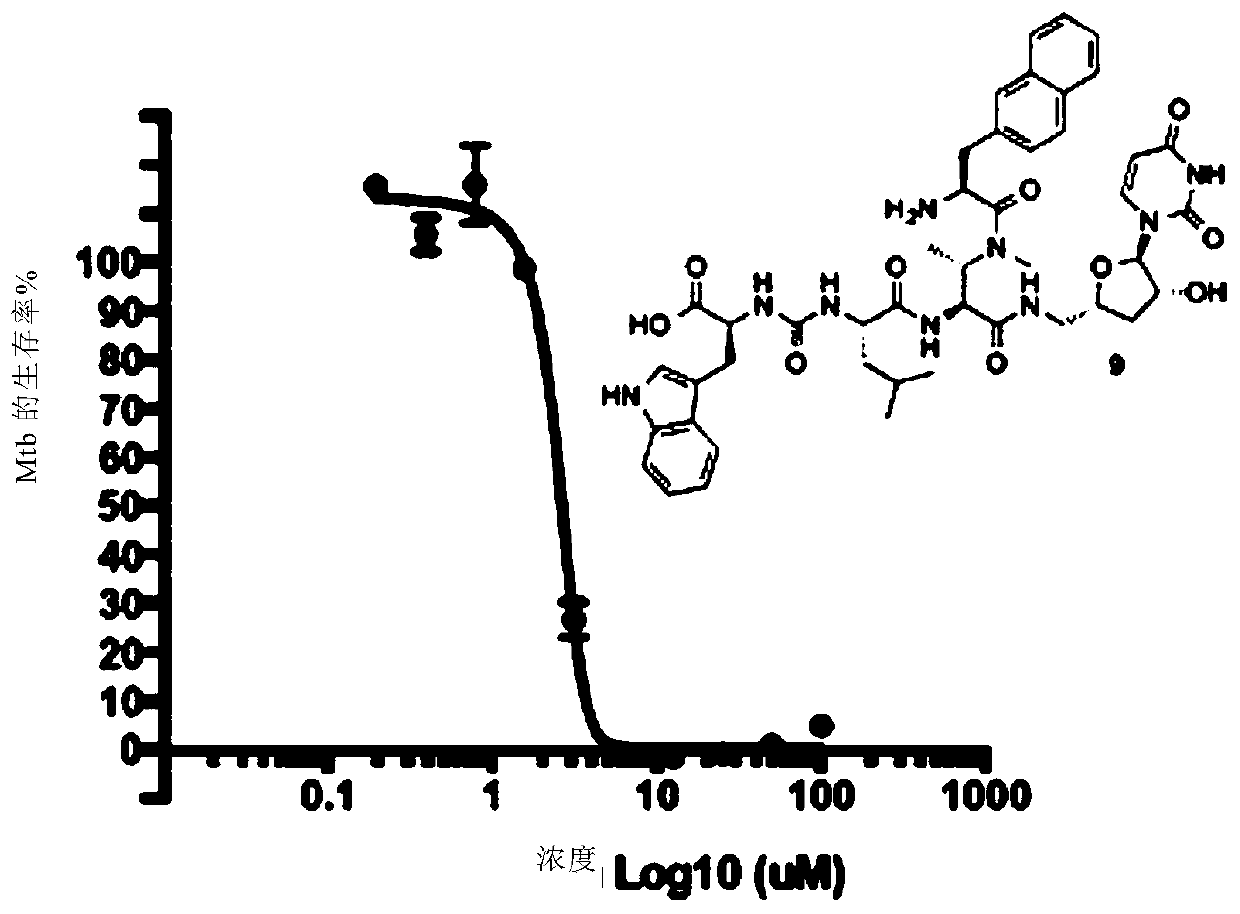
Novel compounds as Anti-mycobacterials
A technology of compounds and solvates, applied in the field of antibacterial compounds, can solve problems such as toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0227] 1. Materials and Methods
[0228] Analytical thin layer chromatography (TLC) was performed on commercially prepared silica plates (Merck Kieselgel 60 0.25 mm F254). Flash column chromatography was performed using 230-400 mesh Kieselgel 60 silica, eluting with distilled solvent as described. The proportions of solvents used for TLC and column chromatography are expressed in v / v. Compounds were visualized by UV light at 254 nm or stained with vanillin or cerium molybdate. Commercial materials were used as is unless otherwise stated. DCM and MeOH were distilled from calcium hydride, THF and diethyl ether were distilled from sodium / benzophenone. tert-butanol Before use, in the activated Dry over molecular sieves for at least 24 hours. Anhydrous DMF was purchased from Sigma Aldrich.
[0229] Device Information
[0230] Unless otherwise stated, at frequencies of 300.2, 400.2, 500.2 and 600.2 MHz, at 300 K, using BrukerAvance DPX 300, DPX 400, DPX 500 and DPX 600 NMR s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com