L-ornithine L-aspartate salt injection solution and preparation method thereof
A technology of ornithine aspartate and injection, which is applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, and medical preparations without active ingredients, etc., can solve the problem of no ornithine aspartate injection. Difficult to guarantee bacterial properties, unknown impurity form and dissolution amount, hidden dangers of drug safety, etc., to achieve excellent drug stability, improve drug safety, and simplify the production process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-7
[0034] Example 1-7 Preparation of Ornithine Aspartate Injection
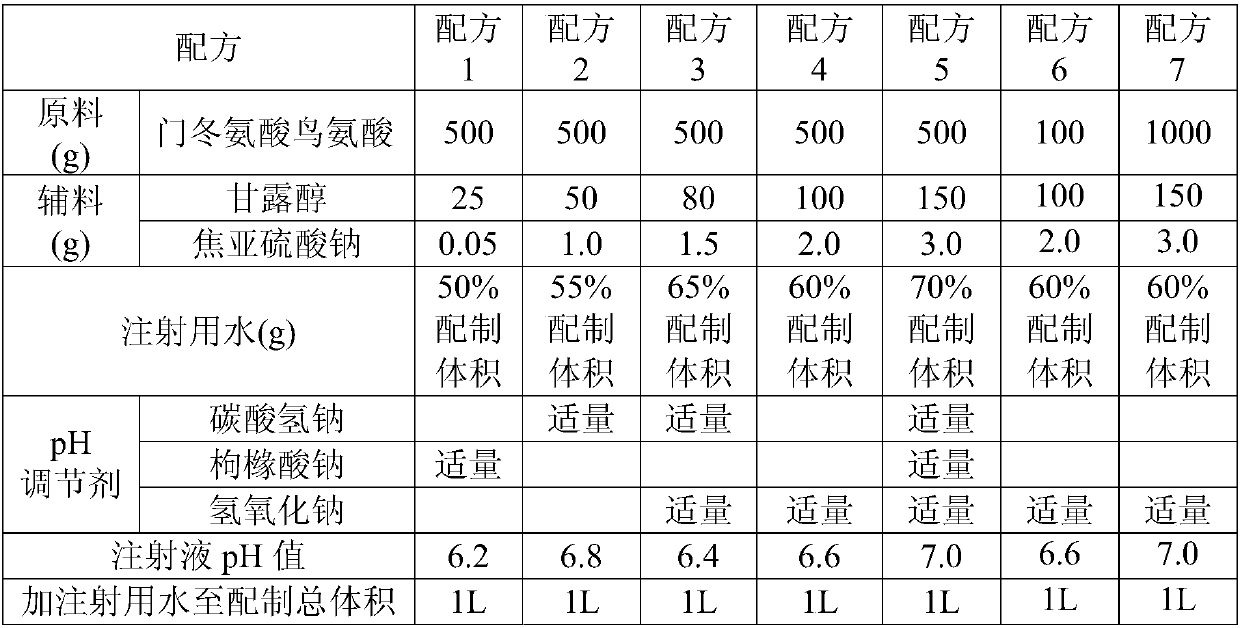
[0035] The formula of ornithine aspartate injection is shown in Table 1.
[0036] Table 1 Ornithine Aspartate Injection Formulation
[0037]
[0038] Specific preparation method
[0039] Prepare Ornithine Aspartate Injection according to the injection formula of Table 1, and the concrete steps are as follows:
[0040] 1) Weigh the mannitol and sodium metabisulfite of the formula amount, dissolve them in 50%~70% of the water for injection of the prepared volume, stir and mix evenly, then add the ornithine aspartate of the formula amount, wait for the ornithine aspartate After all the amino acids are dissolved, adjust the pH with a pH adjuster, control the pH value of the ornithine aspartate injection to be 6.2-7.0, and then add water for injection to make up the total volume.
[0041] 2) The ornithine aspartate injection prepared in step 1) is subjected to sterilization filtration, filling with nitrogen, me...
Embodiment 8
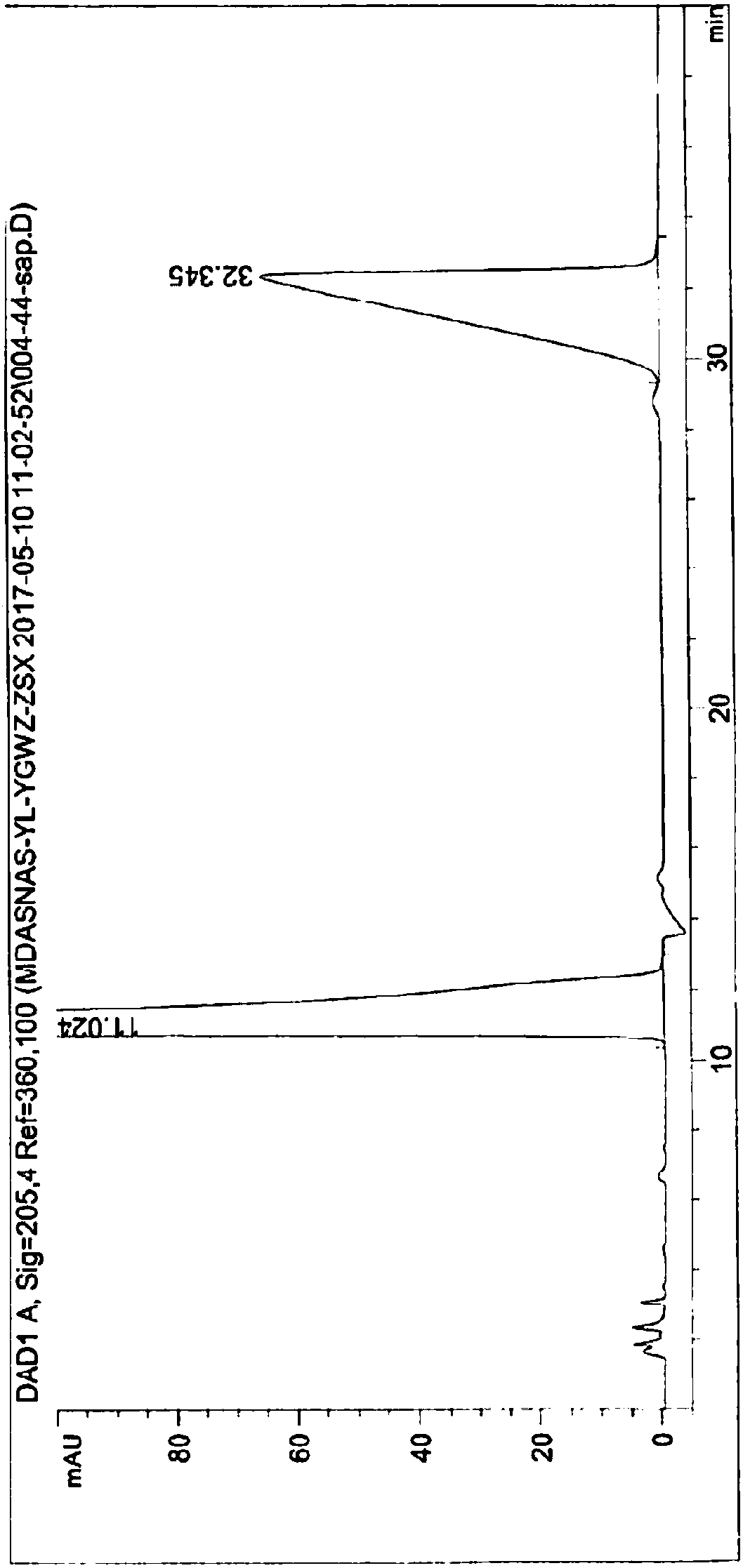
[0046] Embodiment 8, sample stability detection
[0047] In order to investigate the stability of the Ornithine Aspartate Injection of the present invention, the newly prepared Ornithine Aspartate Injection (0 days) and the stability after storage were respectively tested in Examples 1-7.
[0048]Since the conventional storage time is usually 18 months or longer, the present invention stores the Asparagus of the present invention under more severe conditions than the conventional storage conditions (for example: light (4500lx±5001x), high temperature (60°C) conditions) Ornithine amino acid injection, and test the stability of the injection after storage. The details are as follows:
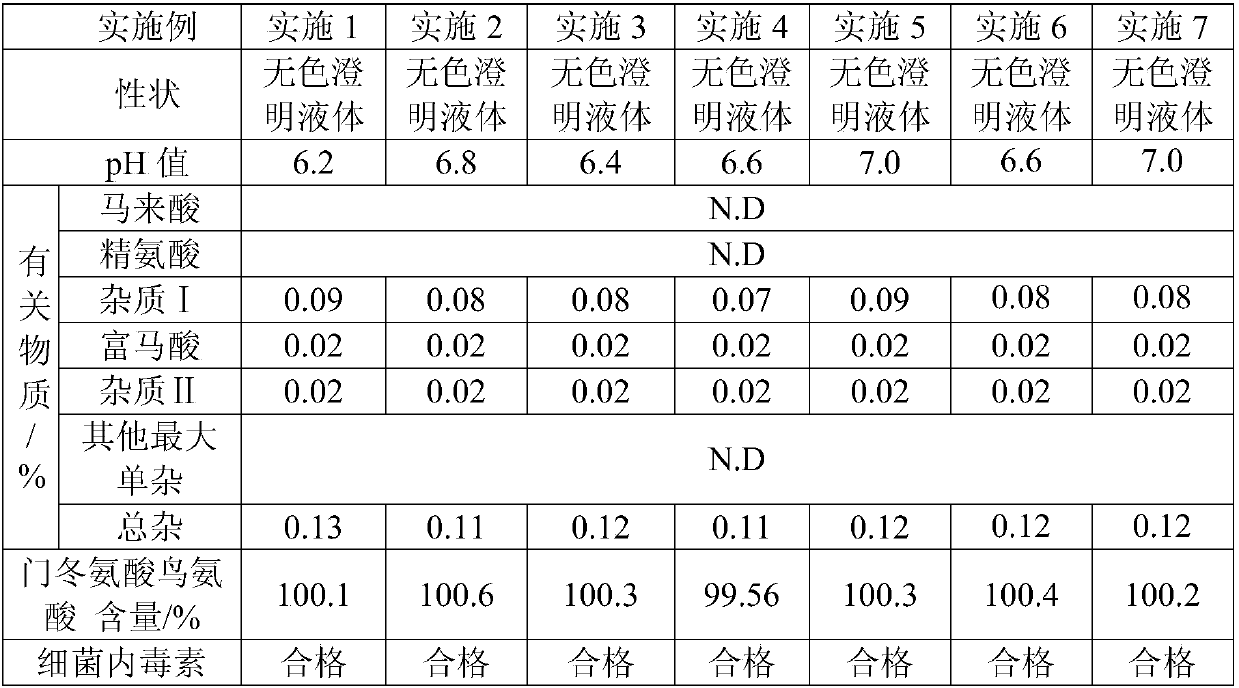
[0049] (1), detect the character, pH value, related substance mass content, ornithine aspartate mass content and bacterial endotoxin of the aspartate ornithine aspartate injection prepared by embodiment 1-7 respectively, test result As shown in table 2.
[0050] Table 2. Test results of ornithi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com