Quinoline-aspidospermine bi-indole alkaloid compound and application thereof
A technology of bisindole biology and quinine, which is applied in the field of quinoline- quinine type bisindole alkaloids and its application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
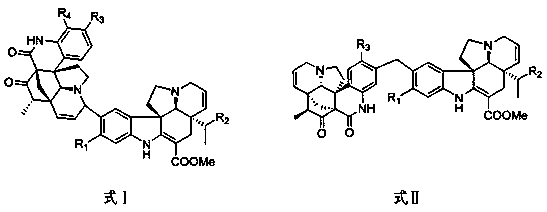
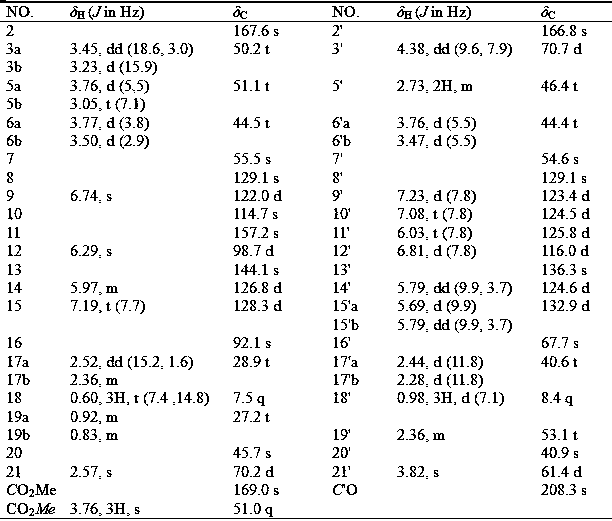
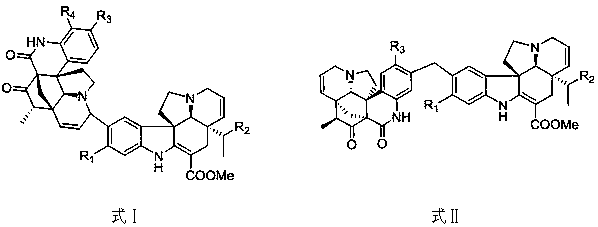
[0020] Example 1: Extraction and separation of quinoline-basindole alkaloids
[0021] A total of 10 kg of Dongshan orange samples were taken, dried and crushed, and then extracted with a methanol solution with a volume concentration of 80% for three times, each time for 3 h. After filtering off the filter residue, the extract was combined to obtain 900g of extract. The extract was used with a volume concentration of 0.2%. Dissolve dilute hydrochloric acid and adjust the pH to 2~3, extract 3 times with ethyl acetate, adjust the pH of the extracted acid solution to 9~10 with 5% by volume ammonia water, extract 3 times with ethyl acetate, and collect The ethyl acetate layer was concentrated to obtain 95g of total alkali fraction; the total alkali fraction was passed through a normal phase silica gel column and eluted with petroleum ether-acetone (volume ratio 100:1, 10:1, 1:1), and the eluate was collected and concentrated Obtain 3 parts (Fr.A, Fr.B, Fr.C);
[0022] Fr.A (14.4g) was ...
Embodiment 2
[0035] Example 2: Inhibition of compounds 1, 2, and 3 on LPS-induced NO release from RAW 264.7 cells
[0036] (1) Material
[0037] DMEM medium (Gibco, USA), fetal bovine serum (Gibco, USA), RPMI-1640 medium (Gibco, USA), phosphate buffer (Shanghai beyotime), double antibody (HyClone, USA), DMSO (USA) Sigma company), MTT (American Sigma company), the compound of the present invention and dexamethasone are all prepared with DMSO;
[0038] (2) MTT method to determine the toxicity of compounds 1, 2, and 3 on RAW264.7 cells
[0039] Prepare a cell suspension with a culture medium (DMEM or RMPI1640) containing 10% fetal bovine serum, and take 200μL of RAW264.7 cell suspension (5×10 4 Pcs / mL) inoculate in 96-well culture plate, in 5% CO 2 Incubate in a constant temperature incubator at 37°C for 24 hours; aspirate the medium inside and add 200 μL of 30 μM test compound solution, and add 200 μL DMEM complete culture solution to the blank control group, with a final volume of 200 μL per well; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com