Quinaine-quinoline type dimer indole alkaloid compound and its application
A technology of dipolybenzdole alkaloids and quercetin, applied in the directions of drug combination, organic chemistry, organic chemistry methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020]Example 1: Extraction Separation of Compound Melokhanine K
[0021]After dry and pulverized, it was dried and pulverized, and the filtration was extracted 3 times with a methanol solution having a volume concentration of 80%, and the filtration was removed and the extract was removed, concentrated under reduced pressure, and the sample containing no organic reagent ( The immersed paste was transferred to a large beaker with an acid water (a volume concentration of 0.5% hydrochloric acid), pH to 2-3, extracted 3 times with ethyl acetate, collecting aqueous layer (alkali moiety) sodium hydroxide The pH is 9-10, then extracted 3 times with ethyl acetate, the ethyl acetate layer was collected, and the ethyl acetate extract was concentrated; the ethyl acetate layer extract was coated with a silica gel column, and the silica gel particle diameter was 100- 200 mesh, gradient with chloroform / acetone solution (volume ratio is 1: 0, 1: 1, 0: 1) elution, the total total of 3 portions were...
Embodiment 2
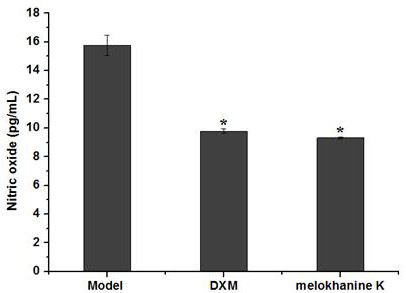
[0028]Example 2: Compound MLOKHANINE K To stimulate macrophages RAW 264.7 to produce NO inhibition in LPS
[0029](1) Experimental method
[0030]Raw 264.7 cell release (2 × 10 per hole is evaluated by detecting the amount of sodium nitrate in the medium by nitrate reductase method.4Cells) allow RAW 264.7 cells to grow on 96-well cell culture plates and in 5% CO2In the environment at 37 ° C for 24 hours; then different concentrations of test samples were added. The test concentration of the test compound of this test was set to 10 μg / ml, 20 μg / ml, 30 μg / ml, and the use of dexamethasone (10 μg / ml). Positive control, LPS as a negative control; continued culture for 2 h, adding LPS (5 μg / ml), continued culture for 24 h, and the culture supernatant was collected; the kit was measured according to nitric oxide (NO) assay kit (nitrate reductase) The method of the method determines the content of NO in the cell, the specific operation is as follows:
[0031]1 Add samples and R1, R2 mixing...
Embodiment 3
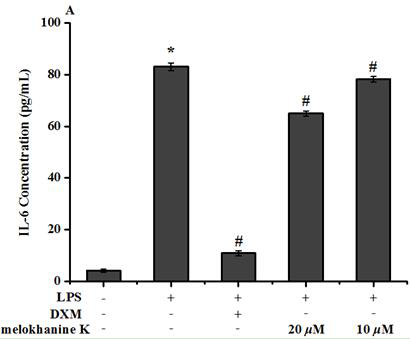
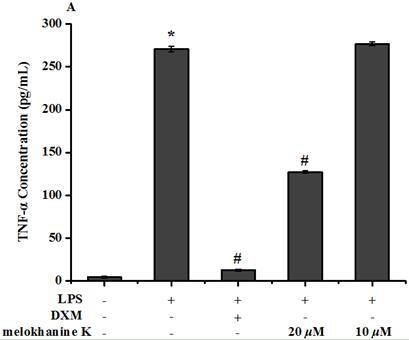
[0039]Example 3: Effect of Compound Melokhanine K on LPS-induced Raw264.7 macrophages induced by LPS
[0040](1) Materials
[0041]DMEM culture solution (US GIBCO company), fetal bovine serum (US GIBCO company), RPMI-1640 culture solution (US GIBCO company), phosphate buffer (Shanghai Beyotime), Double-China Hyclone, DMSO (USA) Sigma, MTT (US SIGMA), the compounds of the invention and dexamethasone were formulated with DMSO.
[0042](2) Preparation of test solution
[0043]It is weighed with 1 mg of compound Melokhanine K, and DMSO is dissolved, and the DMEM is not completely cultured to the desired concentration, and the DMSO final concentration is not more than 0.1%.
[0044](3) method
[0045]Take 200 μl of Raw264.7 cell suspension per well (5 × 104A / mL) was inoculated with a 96-well culture plate. After 24 hours, 10 μl of the test compound dilution was added to the culture solution, and the final concentration of the compound Melokhanine K was 10, 20, 30 μmol / L, each concentration. Three comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com