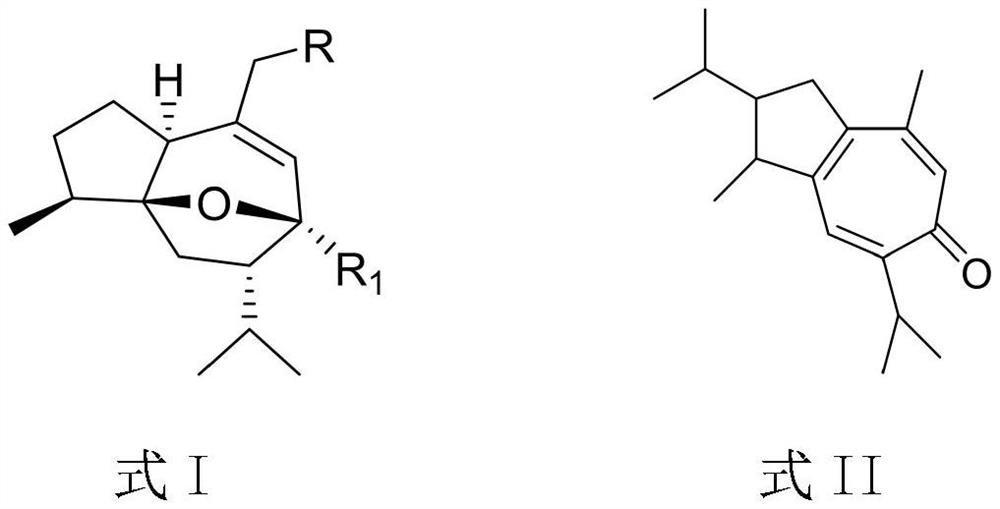
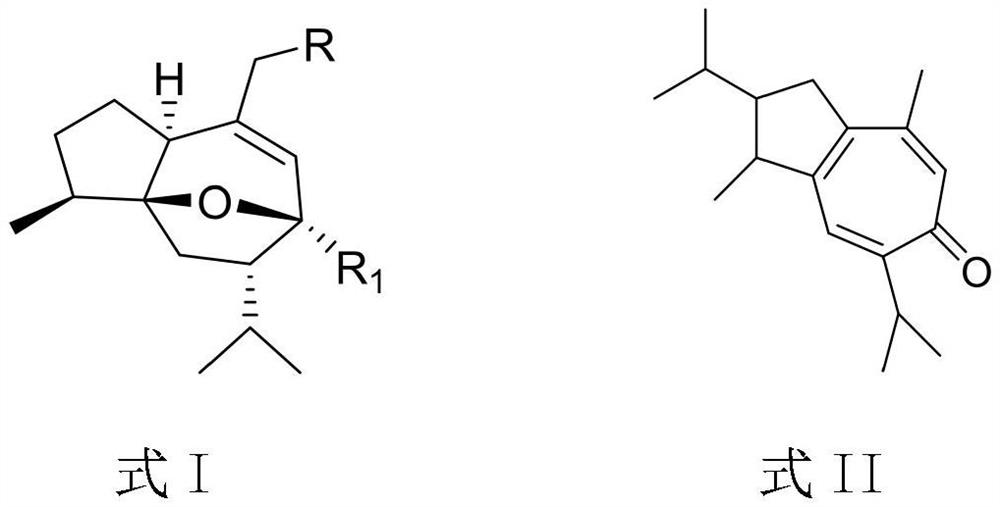
Curcumenol derivative, preparation method and application of curcumenol derivative in preparation of anti-inflammatory drugs
A technology of curcumol and its derivatives, which is applied in the preparation of heterocyclic compounds, anti-inflammatory agents, drug combinations, etc., can solve the problems of aggravating infection and achieve the effect of reducing the level of inflammatory cytokines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
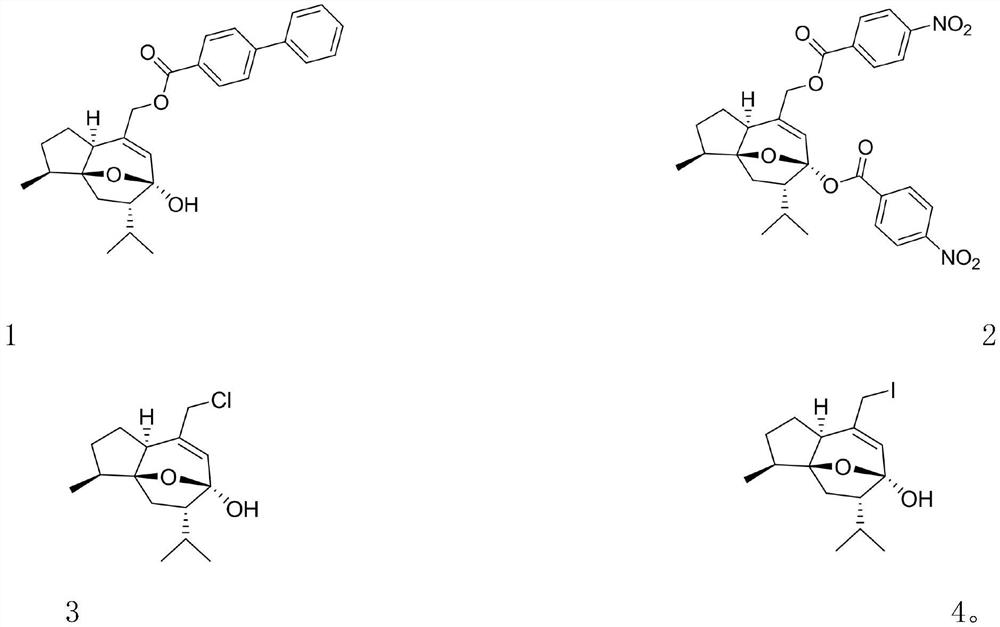
[0036] Synthesis of Curcumol Monoester Derivatives
[0037] Curcumol (3g, 1equiv.) was dissolved in dichloromethane, m-chloroperoxybenzoic acid (3.28g, 1.5equiv.) was added, stirred at room temperature, TLC (5% sulfuric acid ethanol for color development) tracked until the reaction was complete , washed successively with saturated sodium bisulfite, saturated sodium bicarbonate, and saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain an oily liquid, and its oily liquid was dissolved in isopropanol, and sodium hydroxide (1.525 g, 3equiv.), reacted at 70°C, followed by TLC (5% sulfuric acid ethanol for color development) until the reaction was complete, washed with saturated tartaric acid and saturated brine successively, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and passed through column chromatography (petroleum oil) Ether:ethyl acetate=3:1) to isolate 2.242g white solid intermediate (IM), yield...
Embodiment 2
[0041] Synthesis of Curcumol Diester Derivatives
[0042] Synthetic intermediate IM, synthetic method is the same as embodiment 1.
[0043]Dissolve the intermediate IM (50 mg, 1 equiv.) in N, N-dimethylformamide (DMF), add sodium hydride (48 mg, 10 equiv.) and a catalytic amount of potassium iodide in sequence, stir at room temperature for 15 min, and then add p-nitrobenzene Formyl chloride (110 mg, 3 equiv.), stirred at room temperature, followed by TLC (5% sulfuric acid ethanol for color development) until the reaction was complete, quenched with water, washed with saturated tartaric acid and saturated brine successively, dried over anhydrous sodium sulfate, and concentrated under reduced pressure , separated by column chromatography (petroleum ether: ethyl acetate = 15: 1) to obtain 43 mg of white solid, which is the curcumol diester derivative (2), with a yield of 40%.
[0044] 1 H NMR (600MHz, Acetone-d 6 )δ: 8.39 (t, J=8.9Hz, 4H, H-4′, H-6′ and H-4″, H-6″), 8.34–8.29 ...
Embodiment 3
[0046] Synthesis of Chlorine Substituted Derivatives of Curcumol Monoalcohol
[0047] Synthetic intermediate IM, synthetic method is the same as embodiment 1.
[0048] The intermediate IM (50 mg, 1 equiv.) was dissolved in dichloromethane, and N-chlorosuccinimide (NCS) (40 mg, 1.5 equiv.) and triphenylphosphine (PPh3) (78 mg, 1.5 equiv.), stirring at room temperature, followed by TLC (5% sulfuric acid ethanol color) until the reaction was complete, washed with saturated brine, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and column chromatography (petroleum ether: ethyl acetate=15: 1) 43 mg of a colorless transparent liquid was isolated, namely curcumol monoalcohol chlorine-substituted derivative (3), with a yield of 81%.
[0049] 1 H NMR (600MHz, CDCl 3 )δ:5.92(s,1H,H-7),4.01(s,2H,H-9),1.03(d,J=6.7Hz,3H,H-11),1.00(d,J=6.5Hz, 3H,H-12),0.89(d,J=6.6Hz,3H,H-13); 13 C NMR (150MHz, CDCl 3 )δ: 140.06(C-8), 128.75(C-7), 103.27(C-6), 87.44(C-3α), 59....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com