Trinitramine triazolotriazole and its energetic ion salt and its preparation
A technology of triazolo and trinitroamine, which is applied in the directions of nitrated acyclic/alicyclic/heterocyclic amine explosive compositions, organic chemistry, etc., to achieve the effects of high yield, simple synthesis method and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
[0045] The synthesis of embodiment 1 trinitramine three and three (1)
[0046] Add 1.54g of 3,6,7-triamino[1,2,4]triazolo[4,3-b][1,2,4]triazole (10mmol) to 18g of oleum, and wait until the ultrasonic After dissolution, then slowly add 5 mL of fuming nitric acid while keeping the temperature not exceeding -5°C. Reaction for 4h, keeping the temperature at -5°C. It was then quenched with 30 g of crushed ice and filtered to obtain a yellow solid, which was dissolved in CaCl 2 Dry in medium to obtain the product (yield 78%).
[0047] In the present invention, the 3,6,7-triamino[1,2,4]triazolo[4,3-b][1,2,4]triazole is self-made in the laboratory, and the specific preparation method See also T.M. P.C. Schmid, S. Schnell and J. Stierstorfer, Chem. Eur. J., 2015, 21, 9219-9228.
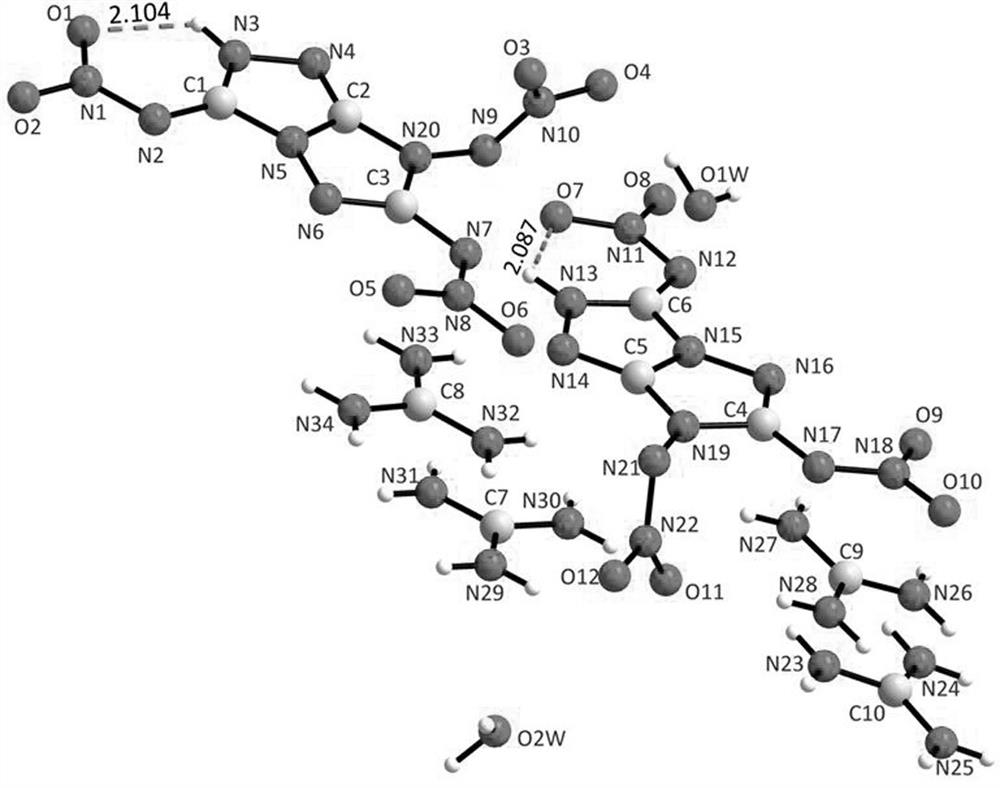
[0048] Product structure identification:
[0049] Decomposition temperature: 89°C (DSC, the following refers to the melting point and decomposition temperature are the initial temperature).
[0050] NMR ...
Embodiment 2 3
[0055] The synthesis of embodiment 2 trinitramine three and three (1)
[0056] Add 1.54g of 3,6,7-triamino[1,2,4]triazolo[4,3-b][1,2,4]triazole (10mmol) into 14g of concentrated sulfuric acid, and wait until it is completely dissolved by ultrasonic Then, keep the temperature not exceeding -5°C, and slowly add 4 mL of anhydrous nitric acid. Reaction for 4h, keeping the temperature at -5°C. It was then quenched with 30 g of crushed ice and filtered to obtain a yellow solid, which was dissolved in CaCl 2 Dry in medium to obtain the product (yield 83%).
Embodiment 3 3
[0057] The synthesis of the double ammonium salt (2) of embodiment 3 trinitramine three three
[0058] Disperse 578 mg of trinitramine trisanetris (2 mmol) in 30 mL of water, add 272 mg of concentrated ammonia water (25% by mass) to it, keep the temperature at 70° C., and stir for 4 h. After cooling, concentrate, filter, and recrystallize in hot water to obtain a yellow solid product (yield 85%).
[0059] Product structure identification, density detection, detonation performance calculation and sensitivity test:
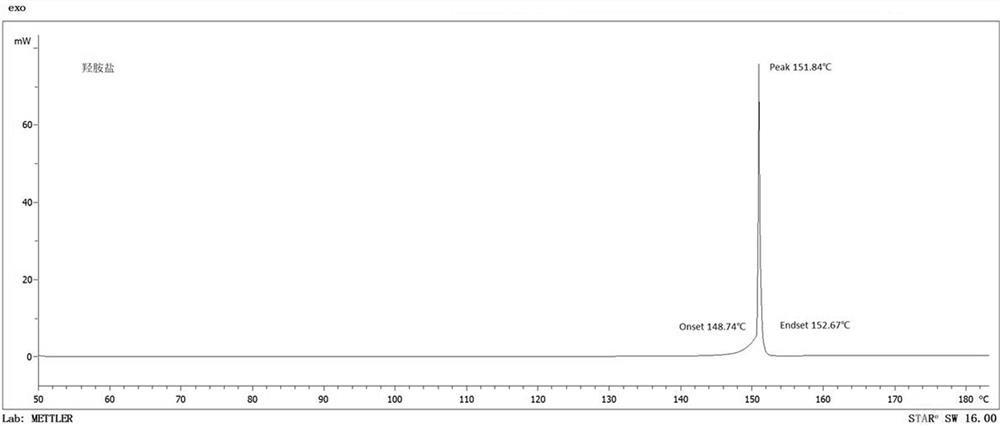
[0060] Melting point: 122°C. The decomposition temperature is 172°C.
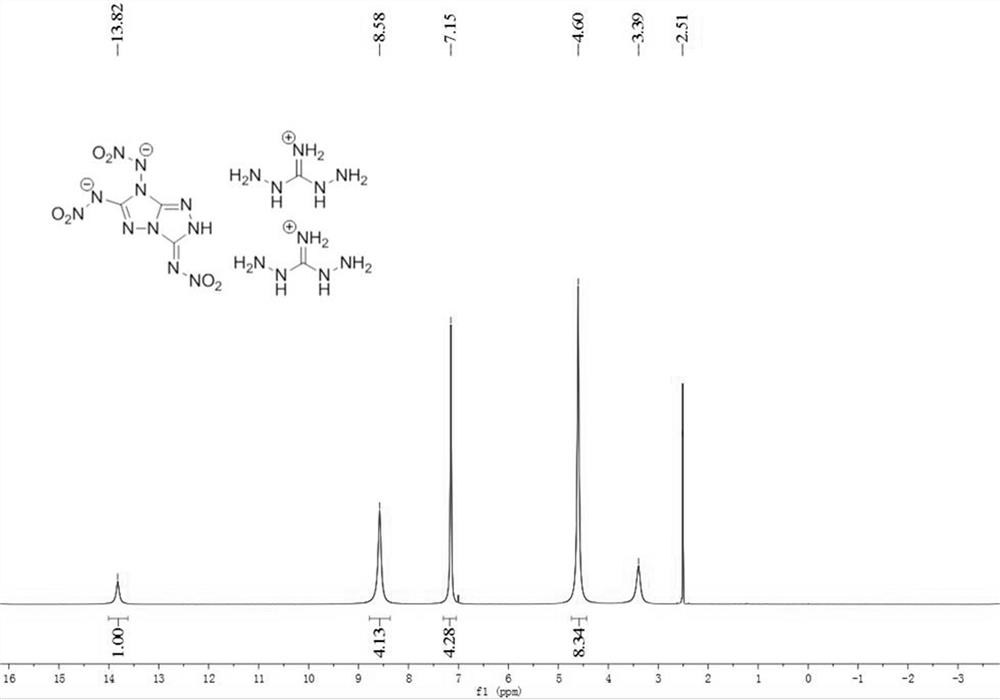
[0061] NMR 1 H NMR ([D 6 ]DMSO, 100MHz, 25°C, TMS): δ=7.14(s)ppm. 13 C NMR ([D 6 ]DMSO, 600MHz, 25°C): δ=159.22, 145.64, 141.85ppm.
[0062] Infrared spectrum (KBr): 3599,3544,3451,3206,1644,1571,1527,1477,1422,1278,1239,1138,1064,1002,952,842,802,764,742,716,582cm -1 .
[0063] Elemental analysis: Molecular formula C 3 h 9 N 13 o 6 , Theoretical C, 11.15; H, 2.81; N, 56.34. Found C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| impact sensitivity | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com