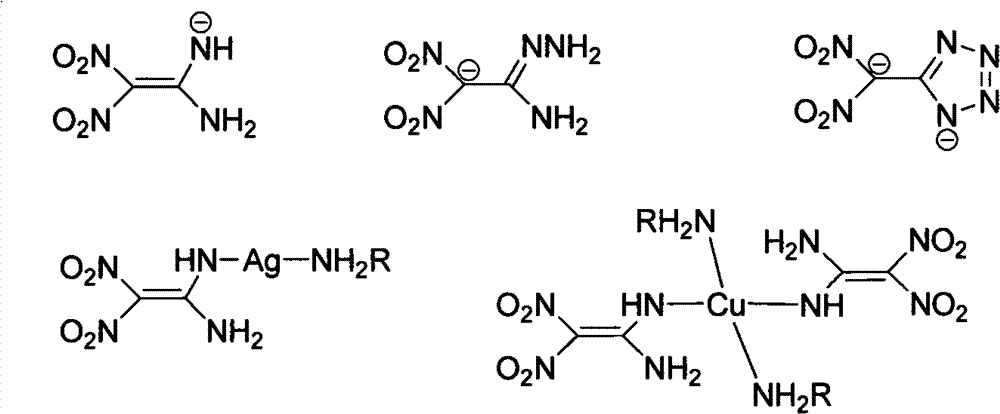
Preparation method and performance calculation for 2-(dinitromethyl)-3-nitro-1,3-diazacyclo-pent-1-ene ionic salt containing energy
A technology of dinitromethyl and dinitromethylene, applied in the direction of nitrated acyclic/alicyclic/heterocyclic amine explosive composition, preparation of urea derivatives, chemical instruments and methods, etc., can solve the problem of nitrogen content Low temperature, poor thermal stability, high detonation velocity, etc., to achieve the effect of simple synthesis method, easy industrialization, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
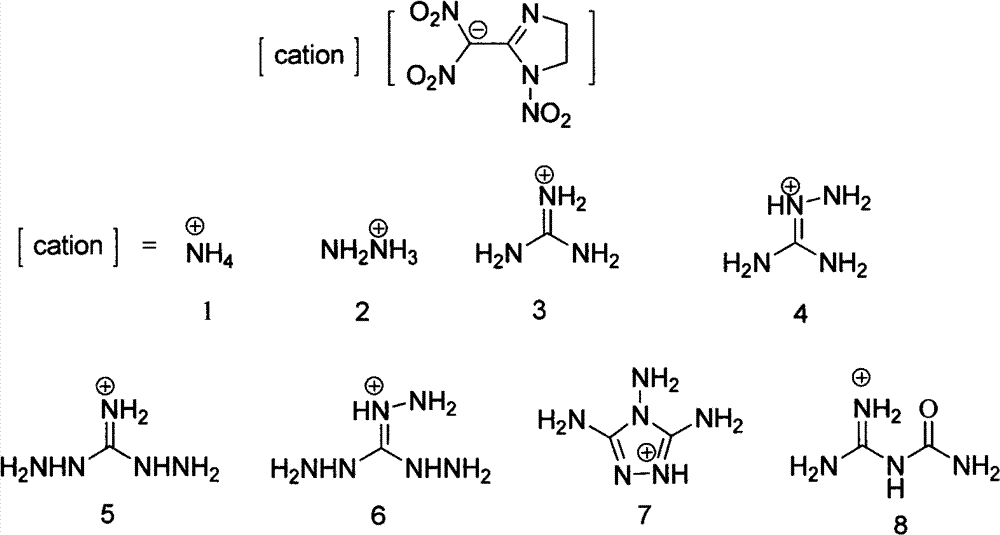
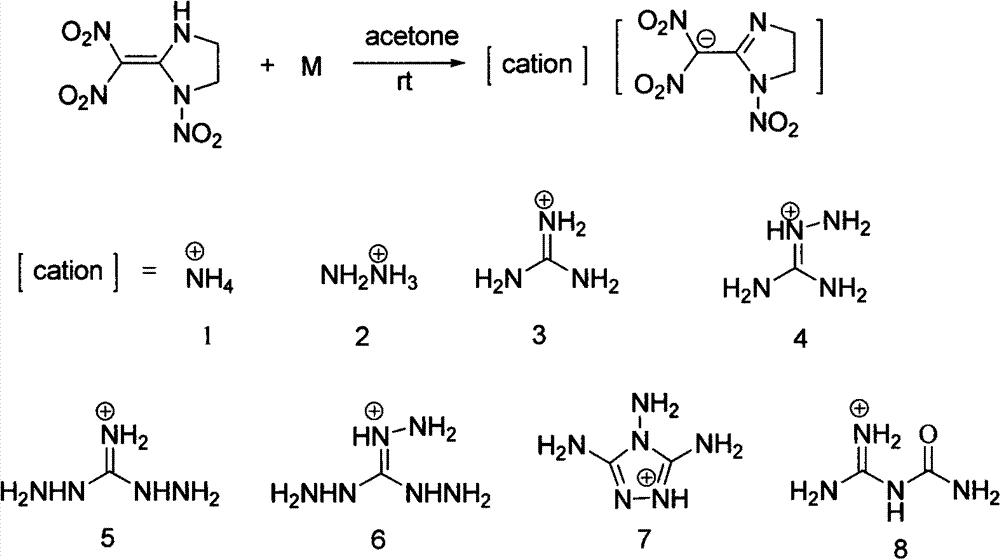
[0027] Add 219mg (1.0mmol) 2-(dinitromethylene)-1-nitro-1,3-diazacyclopentane and 2mL methanol to a 25mL one-necked flask to dissolve into a yellow clear liquid, then stir at room temperature 0.075mL (1.0mmol) 25% ammonia water was added thereto, and the reaction was stirred at room temperature, and a light yellow solid precipitate was separated out. The reaction mixture was stirred at room temperature for 1h, and the light yellow precipitate was filtered and washed twice with dichloromethane and petroleum ether respectively, and the solid After drying in a desiccator at room temperature for 2 hours, 224 mg of the product 2-(dinitromethyl)-3-nitro-1,3-diazacyclopent-1-enyl ammonium salt was obtained, with a yield of 95%.
[0028] The reaction conditions not mentioned in the following examples are the same as in Example 1.
[0029] Its structural formula is as follows:
[0030]
[0031] Decomposition temperature: 158.1°C (DSC). Density is 1.79g cm -3 . 1 H NMR (400MHz, d...
Embodiment 2
[0033] The conditions are the same as in Example 1, except that ammonia water is changed to hydrazine hydrate, and the product 2-(dinitromethyl)-3-nitro-1,3-diazacyclopent-1-ene hydrazine salt yield is 246mg, producing The rate is 98%.
[0034] Its structural formula is as follows:
[0035]
[0036] Decomposition temperature: 143.2°C (DSC). Density is 1.77g cm -3 . 1 HNMR (400MHz, d 6 -DMSO): δ=3.95(t, 2H), 4.19(t, 2H), 7.08(s, 5H) ppm; 13 C NMR (100MHz, d 6-DMSO): δ=48.50, 51.58, 124.42, 148.79ppm; IR (KBr pellet): 3297, 2981, 1614, 1572, 1516, 1498, 1364, 1337, 1287, 1257, 1206, 1169, 1119, 1035, 1007 , 942, 842, 781, 752, 681cm -1 ;elemental analysis(%) calcd for C 4 h 9 N 7 o 6 : C 19.13, H 3.61, N 39.04; found: C 18.86, H 3.36, N 38.96.
Embodiment 3
[0038] Condition is the same as embodiment 1, only changes ammoniacal liquor into the guanidine dissolved in a small amount of methanol, product 2-(dinitromethyl)-3-nitro-1,3-diazacyclopent-1-ene guanidine salt output is 267 mg, 96% yield.
[0039] Its structural formula is as follows:
[0040]
[0041] Decomposition temperature: 173.3°C (DSC). Density is 1.76g cm -3 . 1 H NMR (400MHz, d 6 -DMSO): δ=3.95(t, 2H), 4.20(t, 2H), 6.99(s, 6H) ppm; 13 C NMR (100MHz, d 6 -DMSO): δ=48.11, 51.54, 125.65, 148.33, 158.36ppm; IR (KBr pellet): 3335, 3286, 3135, 1643, 1565, 1535, 1491, 1376, 1241, 1215, 1128, 1004, 533cm -1 ;elemental analysis(%) calcd for C 5 h 10 N 8 o 6 : C 21.59, H 3.62, N 40.28; found: C 19.67, H 3.87, N 41.08.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| impact sensitivity | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com