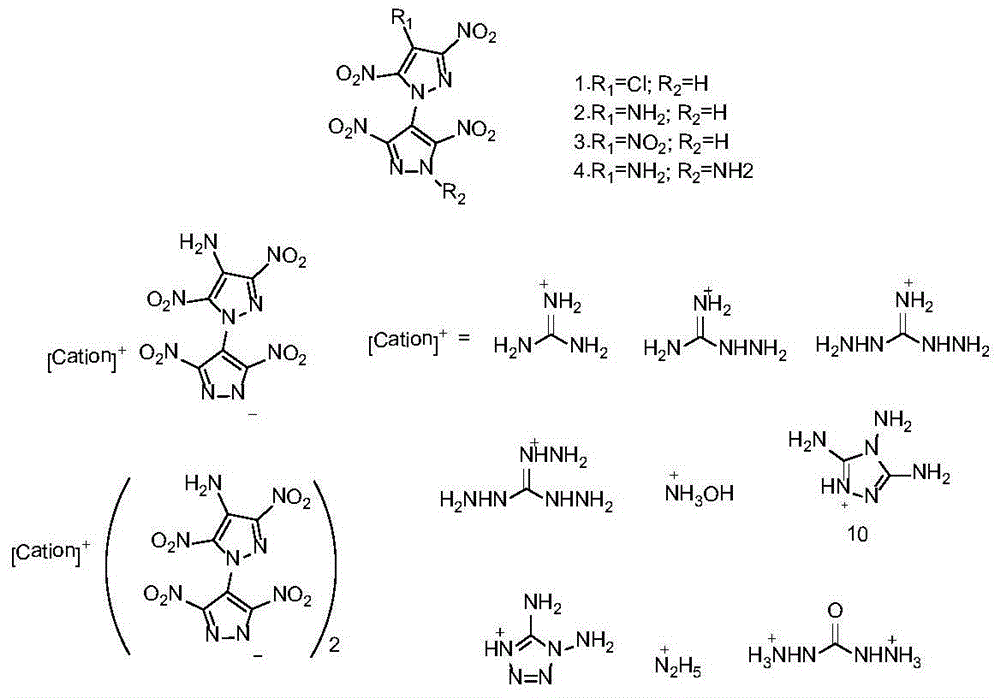
Bispyrazolyl energetic compounds and preparation method thereof
A technology of bispyrazoles and bispyrazoles, which is applied in the field of bispyrazole ring energetic compounds and their preparation, and can solve problems such as poor thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
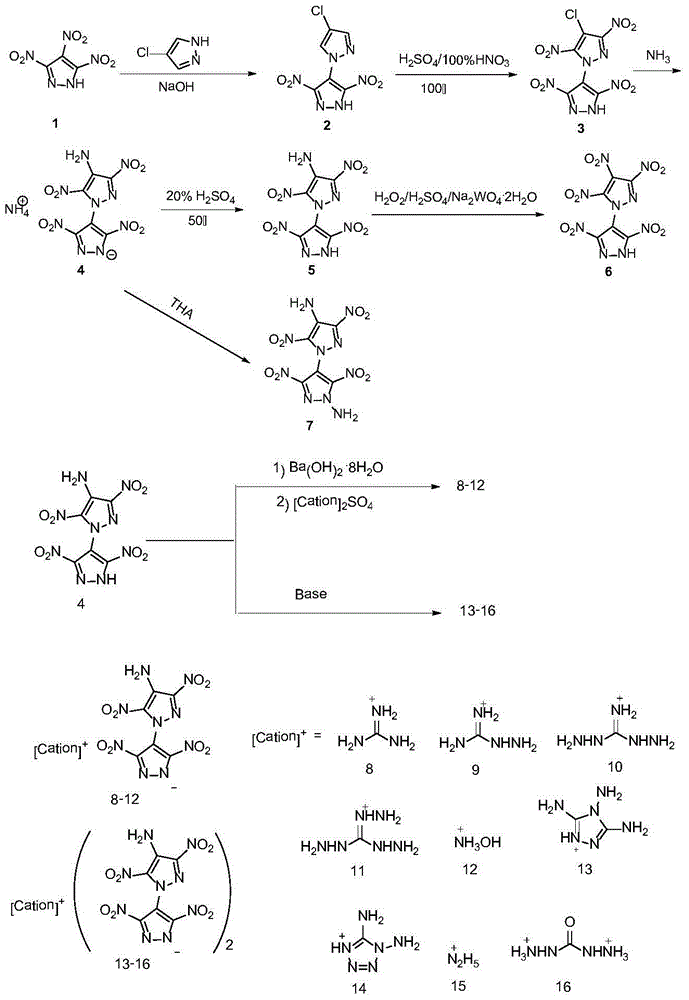
Embodiment 14
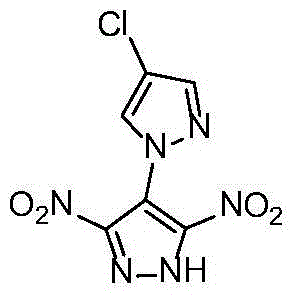
[0027] Example 14 Preparation of chloro-3',5'-dinitro-1'-hydrogen-1,4'-bispyrazole (2)
[0028] Its structural formula is as follows:
[0029]
[0030] Into a 30mL thick-walled sealed glass tube, add 0.203g (1mmol) 3,4,5-trinitropyrazole, 0.102g (1mmol) 4-chloropyrazole, 0.080g (2mmol) NaOH and 2mL water in sequence, and seal Stir, heat up to 160°C, react for 20 hours, cool with 20% H 2 SO 4The solution was acidified to pH=1, filtered to obtain a yellow solid, and recrystallized from water / ethanol to obtain 0.181 g of yellow crystals, with a yield of 62%.
[0031] Melting point: 236°C (DSC, 10K min -1 ). IR(neat):3135,2377,2351,2319,1954,1618,1512,1419,1388,1354,1292,974cm -1 ; 1 H NMR (400MHz, d 6 -DMSO): δ=8.31(s, CH), 7.89(s, CH)ppm; 13 C NMR (400MHz, d 6 -DMSO):δ=147.3,140.4,131.7,112.6,110.5ppm; MS(ESI):m / z(%):257[M-H] - .
Embodiment 24
[0032] Example 2 Preparation of 4-chloro-3,3',5,5'-tetranitro-1'-hydrogen-1,4'-bispyrazole (3)
[0033] Its structural formula is as follows:
[0034]
[0035] Add 0.257g (1mmol) of compound (2) to 3.2mL of concentrated sulfuric acid in batches under ice bath, and slowly add 0.4mL of 100% HNO 3 After the addition, the solution was clear. After removing the ice bath, the temperature was raised to 100° C., and the reaction was carried out for 20 hours, and a large amount of white solid was precipitated. After cooling to room temperature, the reaction solution was slowly added dropwise into 20 mL of ice water with a straw while stirring, and a white solid was precipitated, which was filtered and dried to obtain 0.225 g, with a yield of 65%.
[0036] Melting point: 269°C, decomposition temperature: 308°C (DSC, 10K min -1 ). Density is 1.96g cm -3 . IR (neat):3260,1628,1568,1539,1486,1427,1321,1275cm -1 ; 1 H NMR (400MHz, d 6 -DMSO):δ=6.36(s,NH)ppm; 13 C NMR (400MHz, d ...
Embodiment 33
[0037] Example 33, Preparation of 3′,5,5′-tetranitro-1′-hydrogen-(1,4′-bispyrazole)-4-amine ammonium salt (4)
[0038] Its structural formula is as follows:
[0039]
[0040] Add 0.500g (1.4mmol) of compound (3) and 5mL of 25% ammonia water into a 15mL thick-walled sealed glass tube, stir in a closed chamber, heat up to 80°C, react for 20 hours, filter after cooling, and recrystallize with water to obtain 0.350g of a yellow solid. Yield 70%.
[0041] Decomposition temperature: 262°C (DSC, 10K min -1 ). Density is 1.88g cm -3 . IR(neat):3479,3333,3269,1636,1596,1573,1431,1353,1310,1270,1170cm -1 ; 1 H NMR (400MHz, d 6 -DMSO):δ=7.53(s,2H,NH 2 ), 7.06(s,4H,NH 4 + )ppm; 13 C NMR (400MHz, d 6 -DMSO):δ=150.8,143.0,131.7,130.4,109.7ppm; MS(ESI):m / z(%):328[M] - ;Elemental Analysis: C 6 h 6 N 10 o 8 (346.17) Calculated: C20.82, H1.75, N40.46; Found: C20.97, H1.72, N40.12.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Decomposition temperature | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com