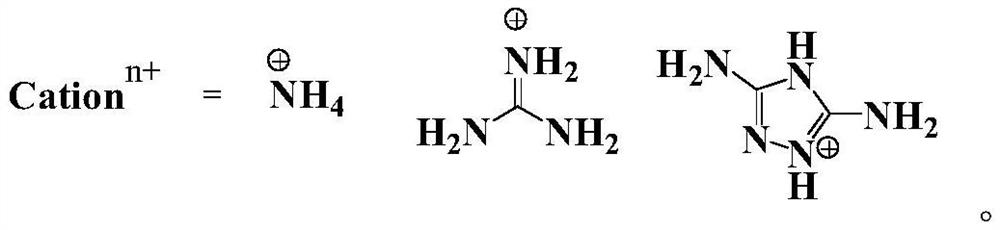
Compounds and their energetic ion salts
A compound and ionic salt technology, applied in the field of energetic material synthesis, to achieve high yield, good thermal stability, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
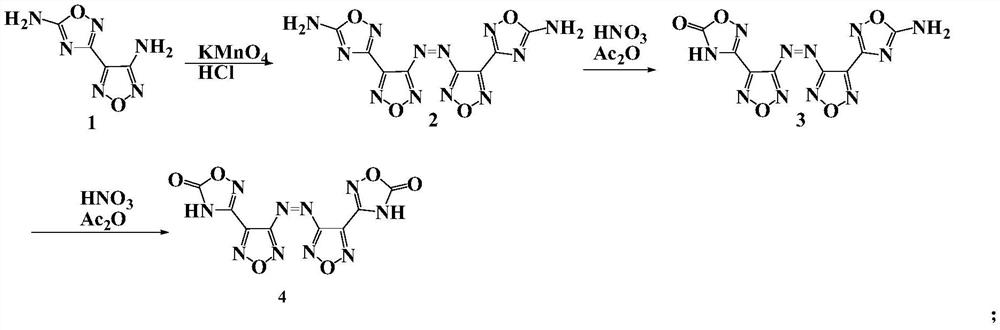
[0032] Preparation of 3,3'-azobis[5-amino-1,2,4-oxadiazol-3-yl-]-1,2,5-oxadiazole, whose structure is as follows:
[0033]
[0034] Compound 1 (4 g, 23.8 mmol) was added to a mixture of acetonitrile (80 ml) and concentrated hydrochloric acid (50 ml) at room temperature. After mixing evenly, slowly add saturated potassium permanganate (3.76g, 23.8mmol) aqueous solution. After the addition, continue stirring for 15min, add water (30ml) for dilution, and slowly add 5% hydrogen peroxide until the solution becomes colorless. After filtering and washing with water, Compound 2 (3.5 g, 10.5 mol) was obtained with a yield of 87.5%.
[0035] Decomposition temperature>250℃. 1 H NMR (500MHz, d6-DMSO): δ8.37ppm. 13 C NMR (500MHz, d6-DMSO): δ173.66, 163.11, 158.33, 142.52ppm.
Embodiment 2
[0037] 4-(5-amino-1,2,4-oxadiazol-3-yl-)-4'-(4H-5-O-1,2,4-oxadiazol-3-yl)-3, The preparation of 3'-azobis 1,2,5-oxadiazole, its structure is as follows:
[0038]
[0039] Compound 2 was oxidized with a mixture of acetic anhydride and 100 wt% nitric acid (mass ratio 2:1) at -20°C-10°C to obtain compound 3.
[0040] 1 H NMR(500MHz,d6-DMSO):δ8.42ppm(s,2H).IR(KBr):υ3450 3280 1770 16701610 1560 1490 1340 1240 1170 1030 973 895 811 764 615 526cm -1 ; Elemental analysis: C 28.83, H 0.92, N 46.26%.
[0041] After differential scanning calorimetry test, the decomposition temperature of compound 3 is 270°C; the measured density is 1.80g / cm -3 Calculated by the equibond equation, the enthalpy of formation is 942.6kJ / mol; calculated by Explo5 software, the detonation velocity is 8419m / s, and the detonation pressure is 29.6GPa; after the sensitivity measurement, the impact sensitivity is 26J, and the friction sensitivity is 240N.
Embodiment 3
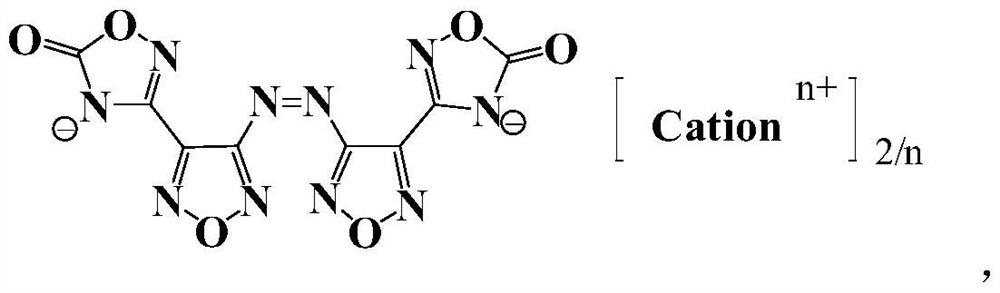
[0043] Preparation of 3,3'-azobis[1,2,4-oxadiazol-5-one-3-yl-]-1,2,5-oxadiazole, whose structure is as follows:
[0044]
[0045]At 0°C, 2 (3.32g, 10mmol) was slowly added to a mixture of acetic anhydride (10ml) and 100% nitric acid (5ml). After reacting at 0°C for 30 minutes, the reaction temperature was raised to room temperature, and the reaction was stopped until solid was precipitated with vigorous stirring. The suspension was poured into ice water, and a large amount of insoluble matter precipitated out. After filtering and washing with water, 4 (1.8 g, 5.4 mmol) was obtained with a yield of 53.9%.
[0046] 1 H NMR (500MHz, d6-DMSO): No peak. 13 C NMR (500MHz, D6-DMSO): Δ162.18,159.52,148.26,139.89ppm.ir (KBR): υ2990 2820 2700 1800 1760 1540 1430 1310 12501140 943 906 873 798686869cm 526C -1 .
[0047] After differential scanning calorimetry test, the decomposition temperature of compound 4 is 322°C; the measured density is 1.89g / cm -3 Calculated by the equibond...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| impact sensitivity | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com