Monoclonal antibody of canine parainfluenza virus, and application thereof
A technology of canine parainfluenza virus and monoclonal antibody, applied in the biological field, can solve the problems of easy missed detection, economic loss, low sensitivity, etc., achieve good reactivity, overcome multiple strains of canine parainfluenza virus or easy to leak The effect of testing positive disease materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Existing product testing situation of embodiment 1
[0047]60 clinically collected eye, nose and throat swabs of suspected canine parainfluenza virus-infected dogs were collected during the epidemiological investigation. RT-PCR, commercial canine parainfluenza virus colloidal gold test strip 1 (Kailing) and commercial test strip 2 (Venture) were used for detection respectively. The results (see Table 1) showed that RT-PCR was positive for 46 1 and 14 were negative, while commercial test strips 1 and 2 detected 21 and 24 positives respectively. The detection rates were high, indicating that the sensitivity of commercial test strips was low.
[0048] Table 1 Clinical sample detection results
[0049] Detection method or product Positive Negative RT-PCR 46 14 Commercial note 1 21 39 commercial note 2 24 36
Embodiment 2
[0050] The separation of embodiment 2 canine parainfluenza virus
[0051] Get RT-PCR detection positive among the embodiment 1, commercialization test strip detection is all negative 22 samples after filtering with 0.22 μm filter membrane, inoculate to about 80% Vero cells, add 10% fetus after absorbing 1 hour DMEM medium with bovine serum, 37°C, 5% CO 2 Continue to cultivate under the same conditions, and observe the cytopathic condition. After blind passage for 3 generations, the virus was harvested. The result: 18 samples had no obvious cytopathic changes after inoculation or the lesions were lost during the passage process, and only 4 samples could produce obvious cytopathic changes after inoculation. The four viral fluids harvested were designed with primers and amplified by molecular biology methods, followed by F gene sequencing. After comparison by NCBI, it was confirmed that they were all canine parainfluenza viruses, and they were named S0419, S0422, and S0427 respec...
Embodiment 3
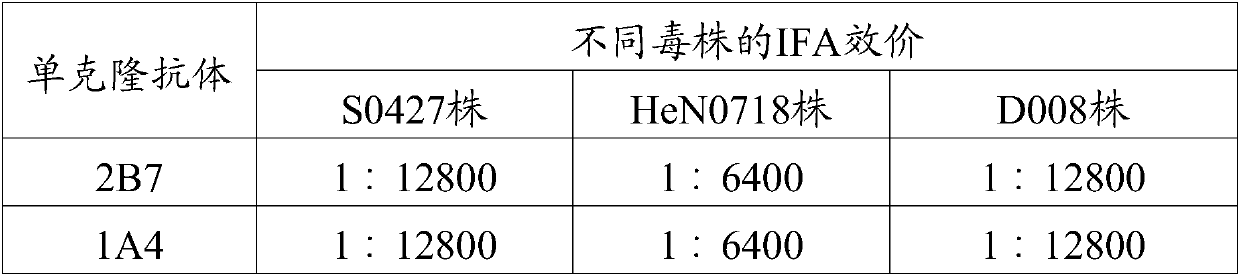
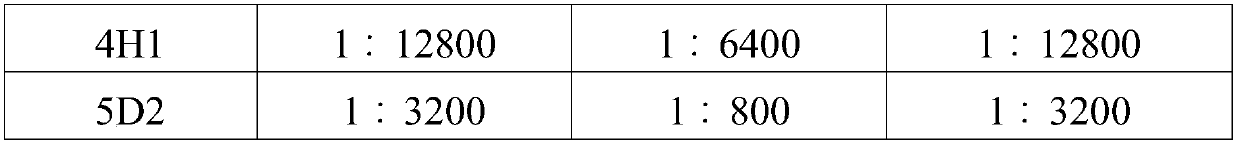
[0053] Example 3 Preparation, Purification and Identification of Canine Parainfluenza Virus Monoclonal Antibody
[0054] 3.1 Immunization of mice
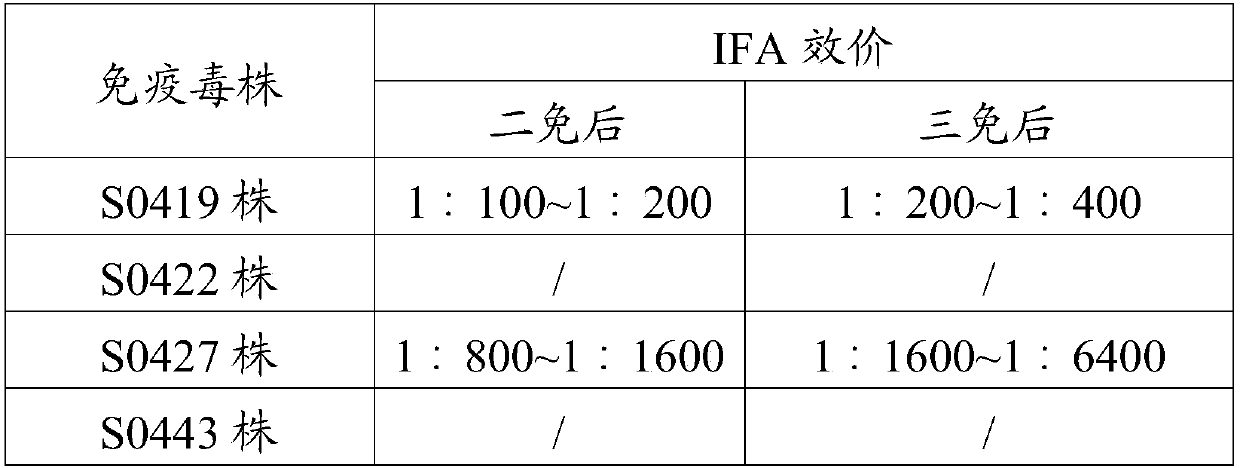
[0055] Four strains of canine parainfluenza virus isolated in Example 2 were used as immunogens, and emulsified in a conventional manner to immunize female BALB / c mice aged 4-6 weeks, 200 μl / mouse. From the second immunization, blood was collected 7 days after each immunization, and the antibody titer of mouse serum after immunization at different stages was detected by IFA method. The results (see Table 2) show that canine parainfluenza virus S0422 strain, S0443 strain 2 strains of virus liquid as immunogen immunized mouse serum does not produce titer, only S0419 strain, S0427 strain of virus liquid immunized mouse serum produces titer , and the IFA titer of the S0427 strain was the highest, so this group of immunized mice was selected for cell fusion to prepare monoclonal antibodies.
[0056] Table 2 Serum IFA titer of immunize...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com