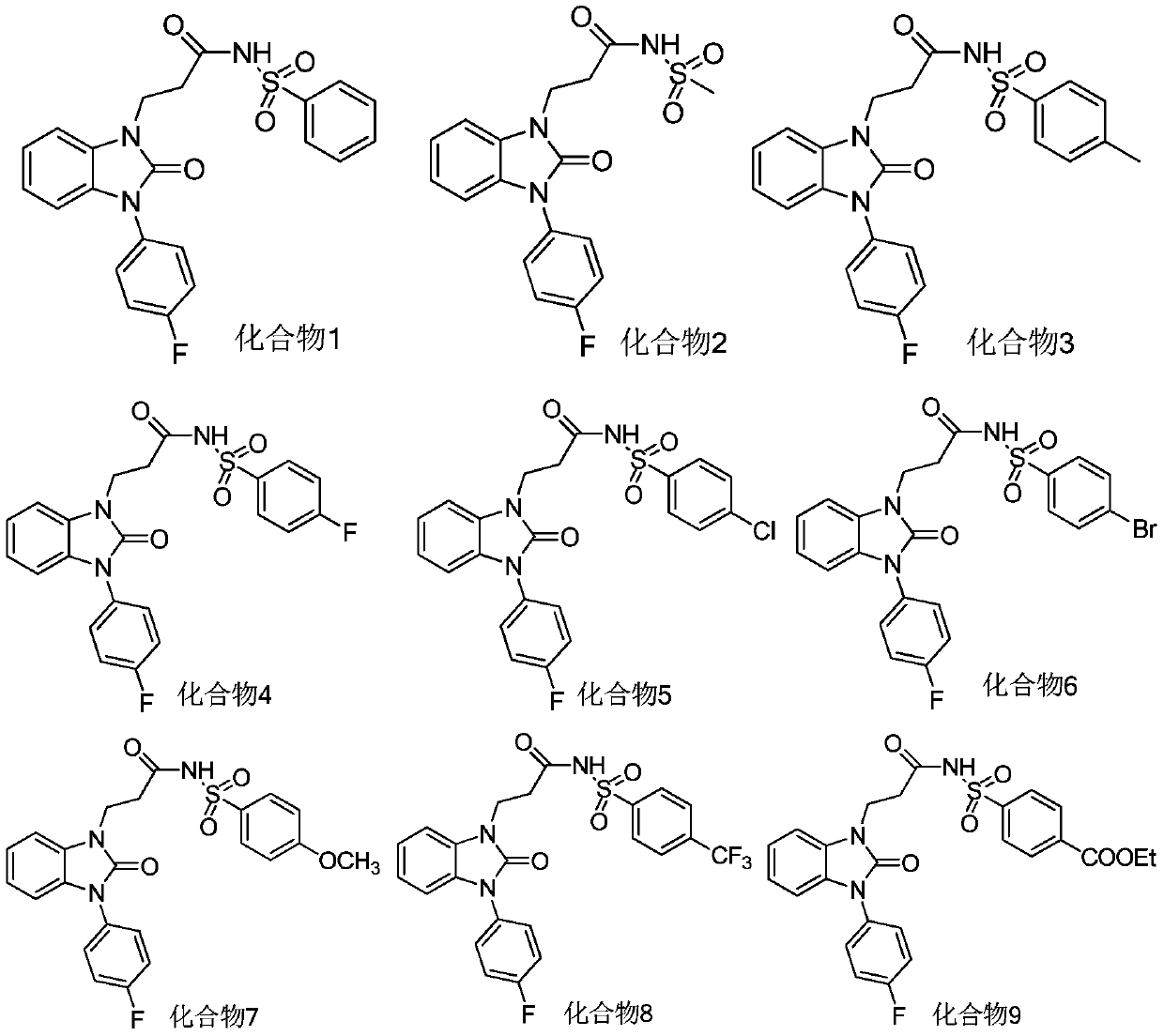
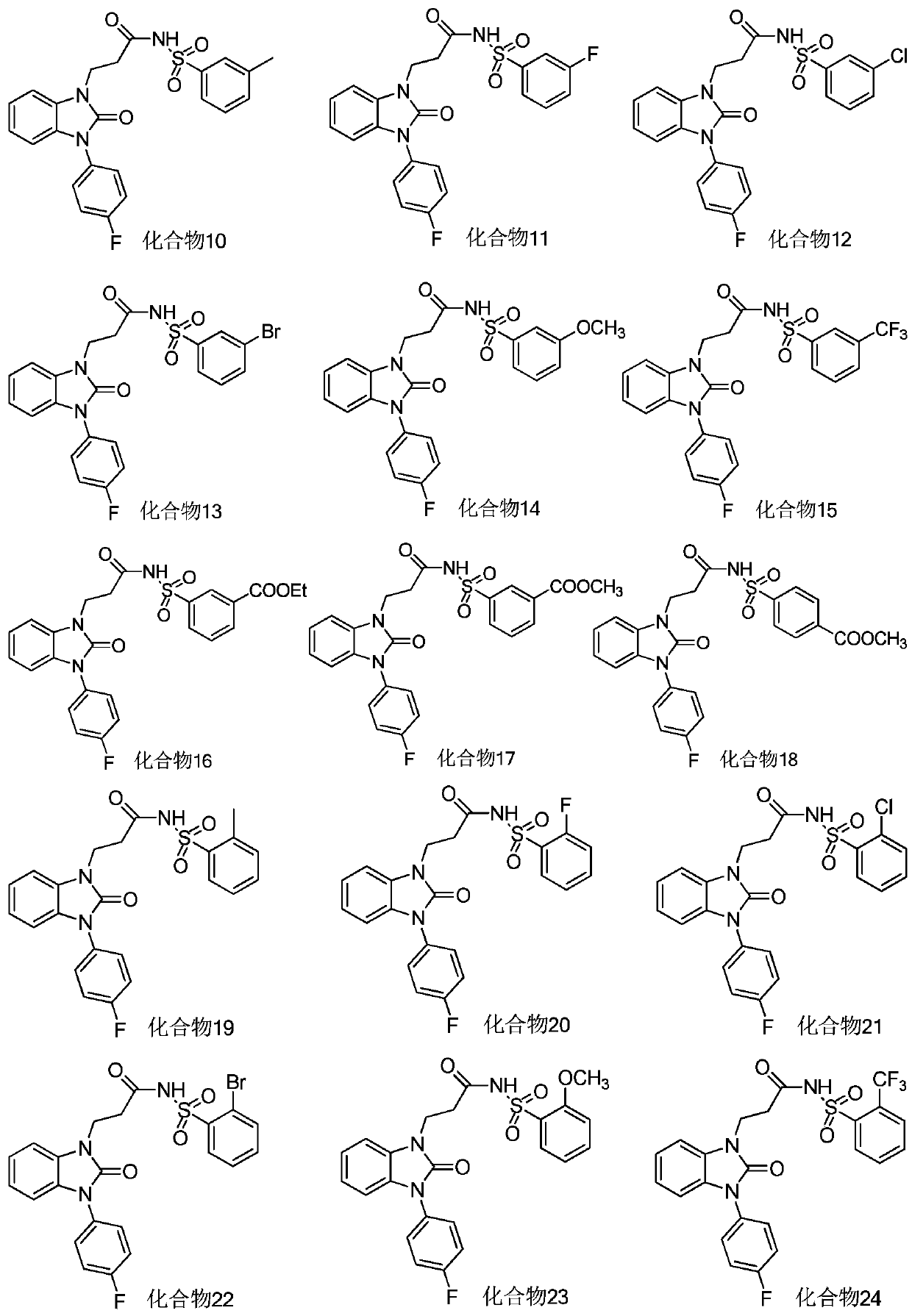
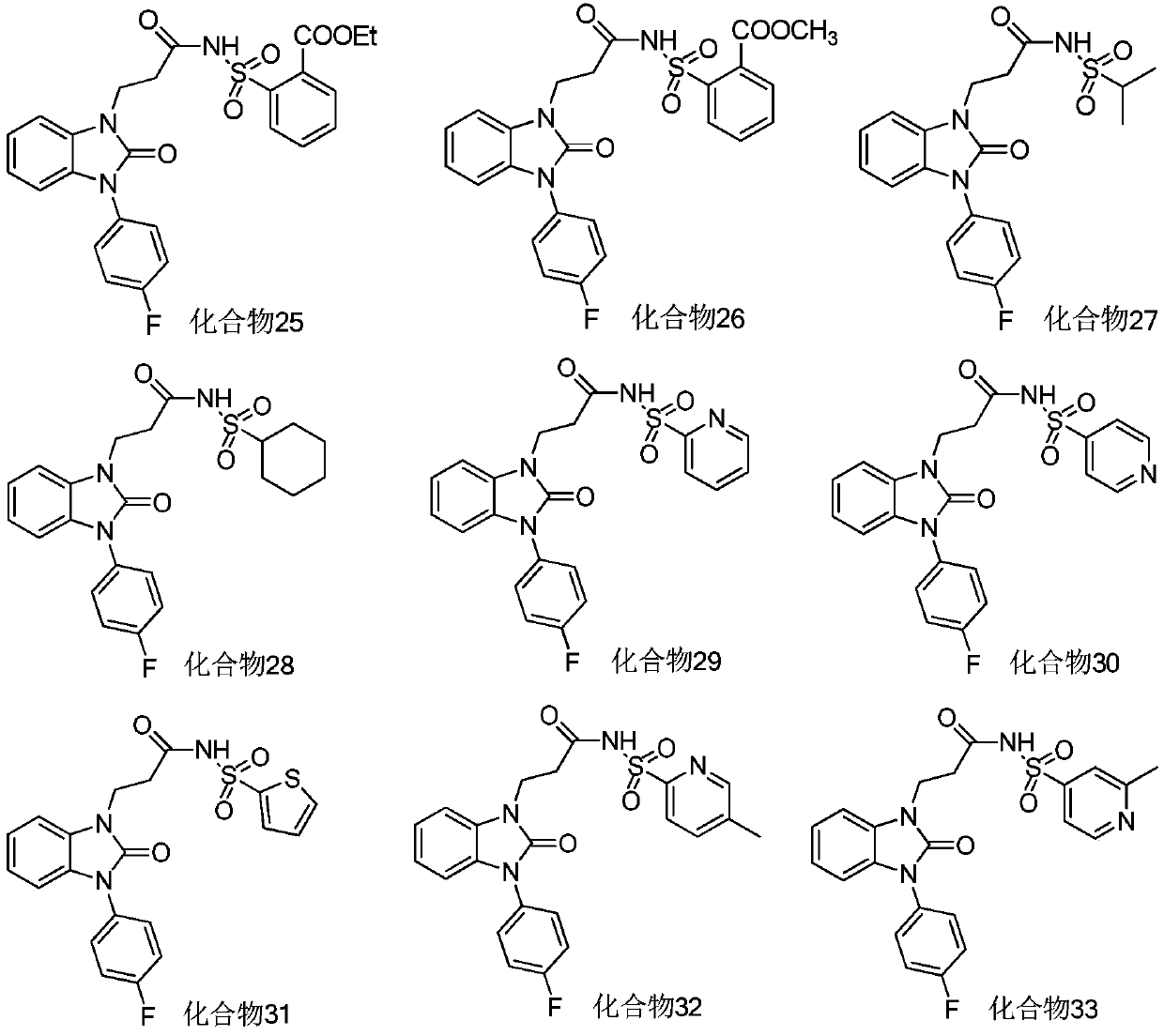
Benzimidazolone compounds, and use thereof in antitussive and antiasthmatic drugs
A technology of benzimidazolone and compound, applied in the field of benzimidazolone compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: the synthesis of intermediate 1
[0019]
[0020] Add methyl 3-(phenylamino)propionate (1 equivalent), palladium acetate (2mol%), silver carbonate (2 equivalents) and dichloroethane (4mL) into a round bottom flask, stir well and then add 4- Fluorophenylisocyanate (1.5 equivalents). The reaction solution was heated at 120° C., and filtered through diatomaceous earth after the completion of the reaction was monitored by TLC. The filtrate was concentrated by rotary evaporation, and the residue was purified by column chromatography (PE / EA=1 / 2) to obtain Intermediate 1 with a yield of 75%. 1 H-NMR (CDCl 3 ,400MHz)δ7.50(dd,J=8.6,4.6Hz,2H),7.21(t,J=8.6Hz,2H),7.17(t,J=7.6Hz,1H),7.08(t,J=7.6 Hz,1H),7.05(d,J=8.0Hz,1H),7.02(d,J=7.6Hz,1H),4.26(t,J=7.0Hz,2H),3.69(s,3H),2.87( t,J=7.0Hz,2H).HRMS(EI)[M+Na] + m / z calcd for C 17 h 15 FN 2 o 3 314.1067,found 314.1066.
Embodiment 2
[0021] Embodiment 2: the synthesis of intermediate 2
[0022]
[0023] Add intermediate 1 (0.1mmoL) to the round bottom flask, add the acetonitrile after dissolving A26(OH - )(1.34mmol / g(wet), 0.2mmoL OH - ). The reaction solution was stirred at room temperature for 16 h and filtered. After washing the resin with acetonitrile, add 20% formic acid solution, stir, and filter. The resin was washed with acetonitrile to obtain the pure intermediate 2 with a yield of 89%. 1 H-NMR (CDCl 3 ,400MHz)δ7.51(dd,J=8.6,4.6Hz,2H),7.21(t,J=8.6Hz,2H),7.18(t,J=7.6Hz,1H),7.08(t,J=7.6 Hz,1H),7.05(d,J=8.0Hz,1H),7.02(d,J=7.6Hz,1H),4.56(t,J=7.0Hz,2H),3.17(t,J=7.0Hz, 2H).HRMS(EI)[M+Na] + m / z calcd for C 16 h 13 FN 2 o 3 300.0910, found 300.0910.
Embodiment 3
[0024] Embodiment 3: the synthesis of compound 1
[0025]
[0026] Add intermediate 2 (1mmoL) and dry THF (1mL) into the round bottom flask, stir to dissolve, add CDI (2.2mmoL) at room temperature, stir the mixture at room temperature for 30min, then heat and stir at 55°C for 1h. After the reaction solution was cooled to room temperature, benzenesulfonamide (2mmoL) was added, and after stirring for 10min, DBU (2mmoL) was added. The reaction solution was stirred at room temperature for 5 h. After the reaction was complete as monitored by TLC, 1.0M HCl (7 mL) was added. The mixture was extracted with water and EA, the combined organic phase was dried over anhydrous sodium sulfate and filtered, the filtrate was concentrated by rotary evaporation, and the residue was purified by column chromatography (PE / EA=2 / 3) to obtain compound 1 with a yield of 72%. 1 H-NMR (CDCl 3 ,400MHz)δ7.79-7.69(m,2H),7.71(dd,J=8.6,4.6Hz,2H),7.51(t,J=8.6Hz,2H),7.42-7.34(m,3H),7.38 (t, J=7.6Hz, 1H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com