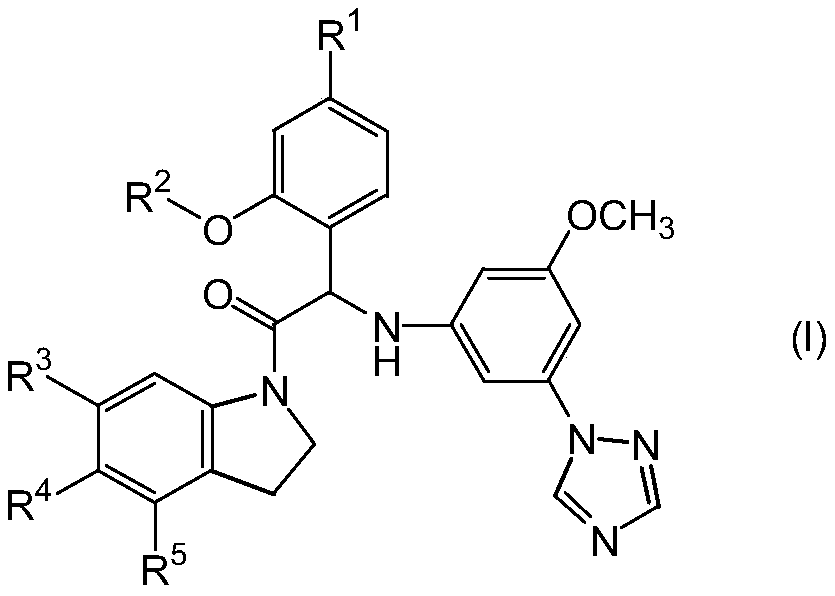
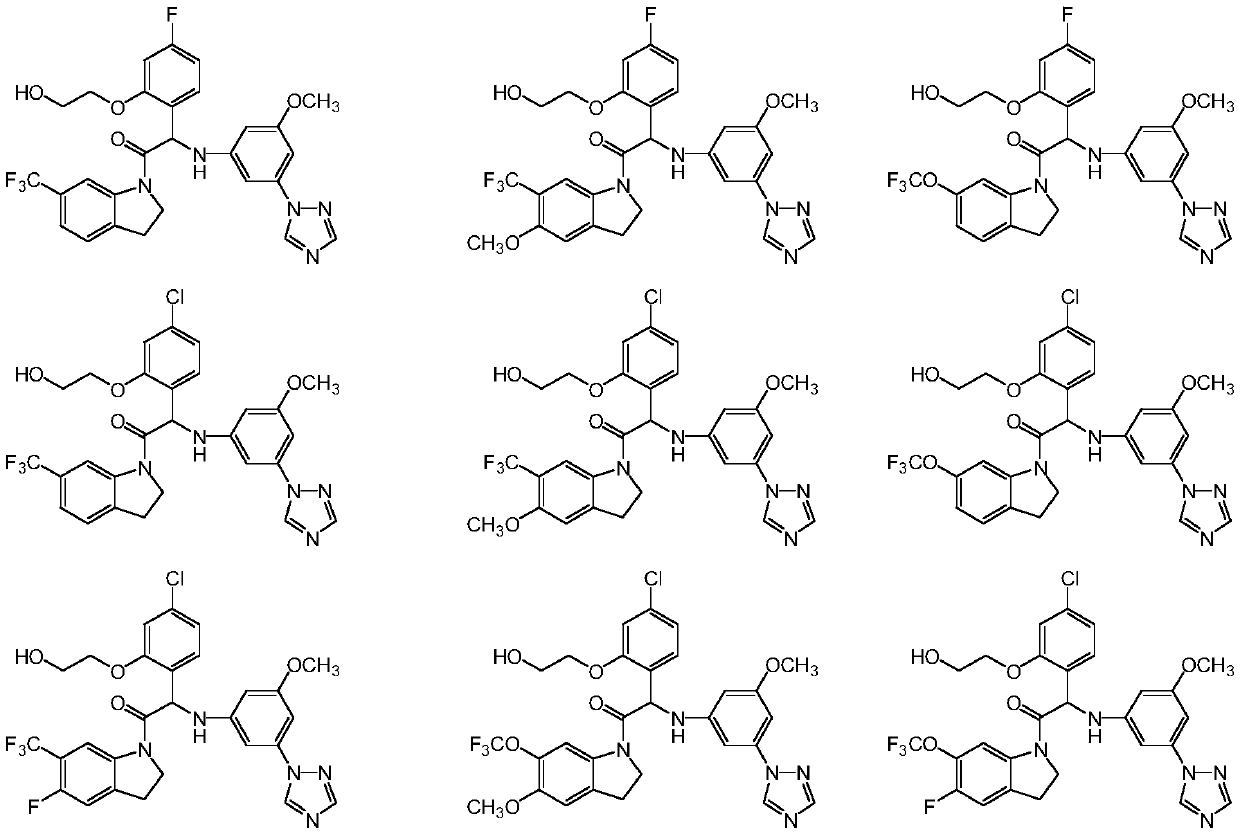
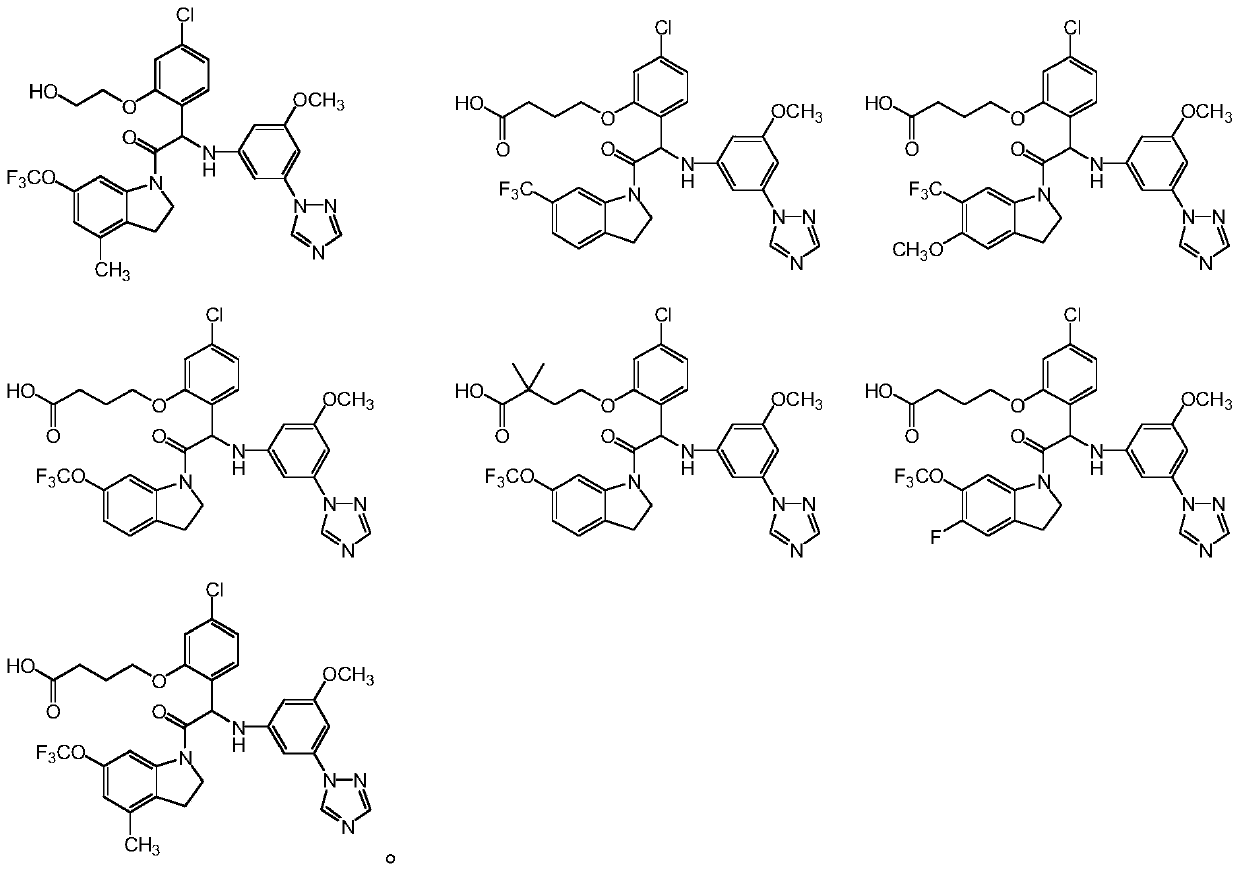
Substituted indoline derivatives as dengue viral replication inhibitors
A technology of dengue fever and solvate, applied in the field of substituted indoline derivatives, can solve the problems of effective, inappropriate, and unavailable specific antiviral drugs for treating or preventing dengue virus infection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0073] Example 1 : 2-(4-fluoro-2-(2-hydroxyethoxy)phenyl)-2-((3-methoxy-5-(1H-1,2,4-triazol-1-yl) Synthesis and chiral separation of phenyl)amino)-1-(6-(trifluoromethyl)indolin-1-yl)ethanone (compound 1) into enantiomers 1A and 1B.
[0074]
[0075] Synthesis of Intermediate 1a:
[0076] 4-Fluoro-2-methoxyphenylacetic acid [CAS 886498-61-9] (10 g, 54.3 mmol) was dissolved in EtOH (200 mL) and H 2 SO 4 (2 mL) was heated at reflux for 12 h. Water was added and the mixture was concentrated under reduced pressure to half the original volume. Add ice. The solution was treated with K 2 CO 3 Basified and extracted with EtOAc. The organic layer was washed with brine, washed with MgSO 4 Dry, filter and concentrate the solvent under reduced pressure to give ethyl 2-(4-fluoro-2-methoxyphenyl)acetate 1a (11.6 g). This compound was used directly in the next step.
[0077] Synthesis of intermediate 1b:
[0078] At -30°C, boron tribromide in CH 2 Cl 2 (109.3 mL, 109.3 mmol)...
example 2
[0108] Example 2 : 2-(4-fluoro-2-(2-hydroxyethoxy)phenyl)-2-((3-methoxy-5-(1H-1,2,4-triazol-1-yl) Synthesis and chiral separation of phenyl)amino)-1-(5-methoxy-6-(trifluoromethyl)indolin-1-yl)ethanone (compound 2) into enantiomers 2A and 2B.
[0109]
[0110] Synthesis of intermediate 2a:
[0111] At -10°C, 1-methoxy-4-nitro-2-(trifluoromethyl)benzene [CAS 654-76-2] (24.5g, 110.8mmol) and 4-chlorophenoxy A mixture of acetonitrile [CAS 3598-13-8] (20.4 g, 121.9 mmol) in DMF (100 mL) was added dropwise to a stirred solution of tBuOK (27.35 g, 243.7 mmol) in DMF (100 mL). After the addition, the purple solution was maintained at -10 °C for 1 h. 500 mL of ice water and 500 mL of 6N HCl were added, and the precipitate was filtered off, washed with water and dried under reduced pressure to obtain 40.4 g of 2-(5-methoxy-2-nitro-4-(trifluoro Methyl)phenyl)acetonitrile 2a (used as such in next step).
[0112] Synthesis of intermediate 2b:
[0113] 2-(5-Methoxy-2-nitro-4-(tri...
example 3
[0135] Example 3 : 2-(4-fluoro-2-(2-hydroxyethoxy)phenyl)-2-((3-methoxy-5-(1H-1,2,4-triazol-1-yl) Synthesis and chiral separation of phenyl)amino)-1-(6-(trifluoromethoxy)indolin-1-yl)ethanone (compound 3) into enantiomers 3A and 3B.
[0136]
[0137] Synthesis of intermediate 3a:
[0138] To 2-(2-(2-((tert-butyldimethylsilyl)oxy)ethoxy)-4-fluorophenyl)-2-((3-methoxy-5-(1H - To a solution of 1,2,4-triazol-1-yl)phenyl)amino)acetic acid 1f (1.02g, 1.974mmol) in DMF (10mL) was added HATU (1.13g, 2.96mmol), diisopropyl Ethylamine (979 μL, 5.92 mmol) and 6-(trifluoromethoxy)indoline [CAS 959235-95-1] (401 mg, 1.97 mmol). The reaction mixture was stirred at room temperature for 2 h. The reaction mixture was diluted with water. The precipitate was filtered off, washed with water and taken up with EtOAc. The organic layer was washed with K 2 CO 3 10% solution in water, washed with water, over MgSO 4 Dry, filter and concentrate the solvent under reduced pressure to give 2-(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com