Preparation method of 5-hydroxytryptophan sustained-release tablets
A technology of hydroxytryptophan and controlled-release tablets, which can be applied in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., can solve the problem of high cost of 5-hydroxytryptophan matrix sustained-release tablets , to achieve the effects of good production controllability, relieving depression and increasing wear resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
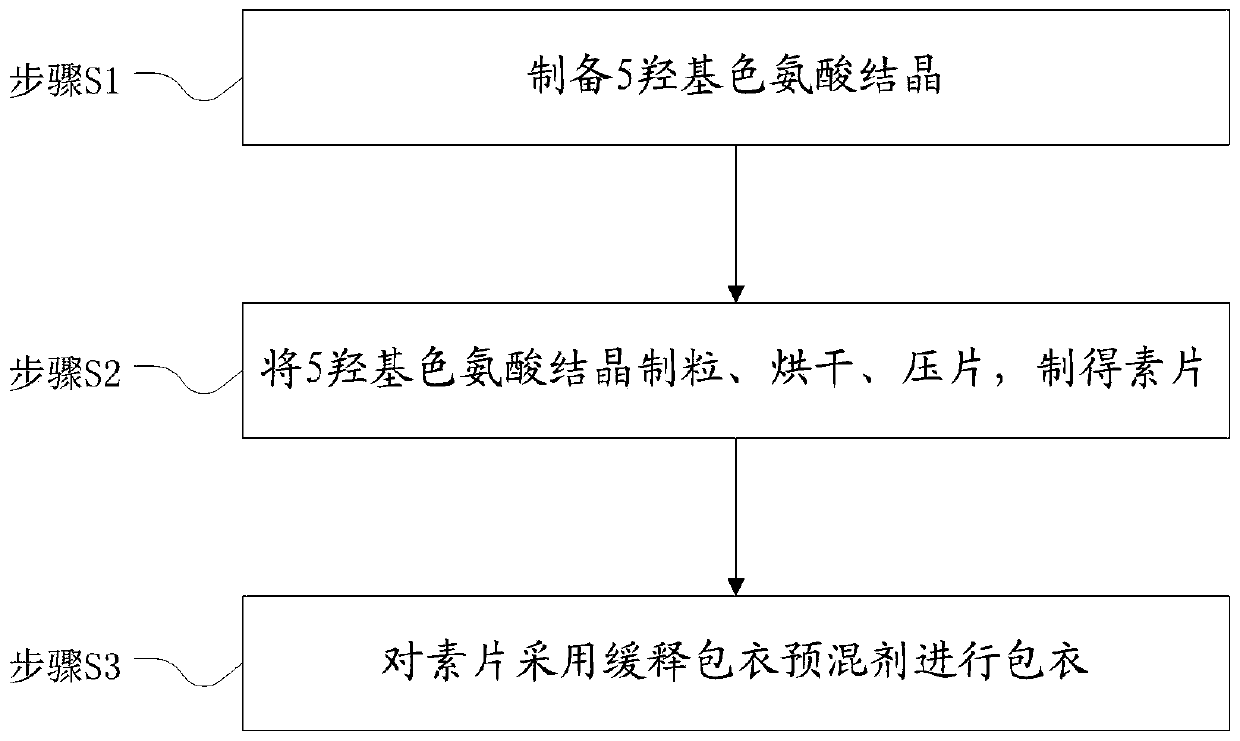
[0032] The preparation method of 5-hydroxytryptophan sustained and controlled release tablets, see the flow chart figure 1 , including the following steps:
[0033] Step S1, preparing 5-hydroxytryptophan crystals;
[0034] Step S2, granulating, drying, and tableting 5-hydroxytryptophan crystals to obtain a plain tablet; the process of preparing the plain tablet can use the preparation method in the prior art;
[0035] Step S3, coating the plain tablet with a slow-release coating premix, and adjusting the dosage of the slow-release coating premix according to the release rate to achieve the expected release rate;
[0036] Wherein the sustained-release coating premix comprises, by weight: 30 parts of ethyl cellulose, 2 parts of silicon dioxide and 1 part of walnut oil.
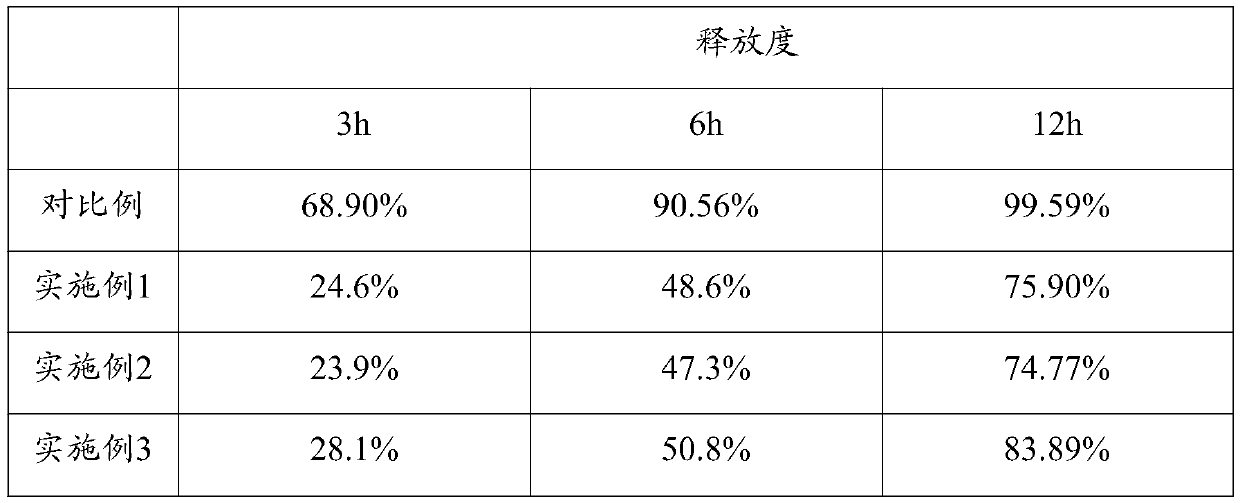
[0037] During the coating process, the weight ratio of the final coating to the plain tablet is controlled between 1:25 and 1:12, and a batch of several 5-hydroxytryptophan sustained-release tablets are prepar...
Embodiment 2
[0039] The preparation method of 5-hydroxytryptophan sustained and controlled release tablet comprises the following steps:
[0040] Step S1, preparing 5-hydroxytryptophan crystals;
[0041] Step S2, granulating 5-hydroxytryptophan crystals, drying, and tableting to obtain plain tablets;
[0042] Step S3, coating the plain tablet with a slow-release coating premix, and adjusting the dosage of the slow-release coating premix according to the release rate to achieve the expected release rate;
[0043] The sustained-release coating premix comprises, by weight, 70 parts of ethyl cellulose, 8 parts of titanium dioxide, and 6 parts of a mixture of rapeseed oil and soybean oil.
[0044] During the coating process, the weight ratio of the final coating to the plain tablet is controlled between 1:25 and 1:12, and a batch of several 5-hydroxytryptophan sustained-release tablets are prepared.
Embodiment 3
[0046] The preparation method of 5-hydroxytryptophan sustained and controlled release tablet comprises the following steps:
[0047] Step S1, preparing 5-hydroxytryptophan crystals;
[0048] Step S2, granulating 5-hydroxytryptophan crystals, drying, and tableting to obtain plain tablets;
[0049] Step S3, coating the plain tablet with a slow-release coating premix, and adjusting the dosage of the slow-release coating premix according to the release rate to achieve the expected release rate;
[0050] The slow-release coating premix comprises, by weight, 50 parts of ethyl cellulose, 5 parts of plant carbon black, a mixture of yellow iron oxide and 4 parts of sesame oil.
[0051] During the coating process, the weight ratio of the final coating to the plain tablet is controlled between 1:25 and 1:12, and a batch of several 5-hydroxytryptophan sustained-release tablets are prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com