Low-dielectric-constant solvent for injection and application thereof
A low dielectric constant, injection technology, applied in the field of medicine, can solve the difficult problems of Xuesaitong injection stability, high adverse reaction reports, quality changes during random inspection, etc., to achieve relaxation of quality requirements and product transportation and storage , good industrial applicability, and the effect of reducing production control costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] The method for preparing Xuesaitong injection prepared with a low dielectric constant solvent for injection of the present invention includes the following steps:
[0060] method one:
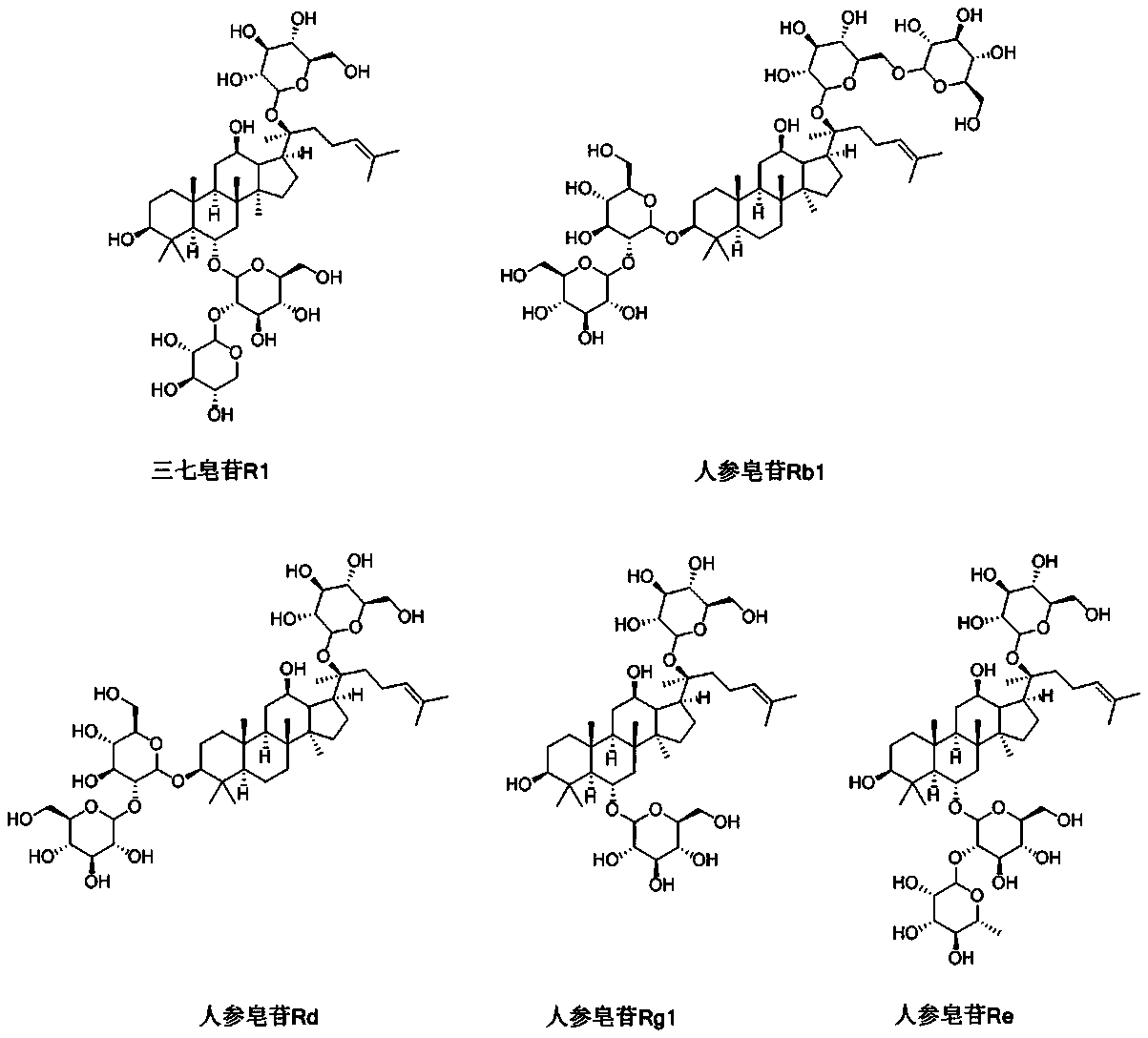
[0061] 1) Concentration: Heat 40-60% of the propylene glycol in the formula to 50-60°C, add the sodium chloride in the formula, keep stirring until the dissolution is complete, and then add the panax notoginseng saponins in the formula , Stir continuously until the dissolution is complete, add activated carbon with a mass percentage of 10-15% of the total saponins of Panax notoginseng. After stirring, let it stand for 20-40 minutes to decarbonize and obtain a concentrated liquid a;
[0062] 2) Dilute preparation: add the remaining 40-60% of the propylene glycol in the formula a to the concentrated solution a, stir and add 2~4% activated carbon of the total saponins of notoginseng saponins. After stirring, let it stand for 20-40min to remove the carbon. , Fine filtration to obtain liquid medici...
Embodiment 1
[0077] After heating 1500 g of propylene glycol to 55° C., add 25 g of sodium chloride and stir continuously until the dissolution is complete. Add 170g of panax notoginseng saponins and keep stirring until the dissolution is complete. Add 24 g of activated carbon, stir and let it stand for 30 minutes to decarburize to obtain a concentrated medicine. Add 1500 g of propylene glycol to the concentrated medicine, stir and add 6 g of activated carbon, stir and let stand for 30 minutes, decarburize, and finely filter to obtain a liquid medicine. Fill the liquid medicine into the ampoule and seal it. Then sterilize at 100°C for 30 minutes, and perform lamp inspection. Finally, the product is packaged according to the specifications.
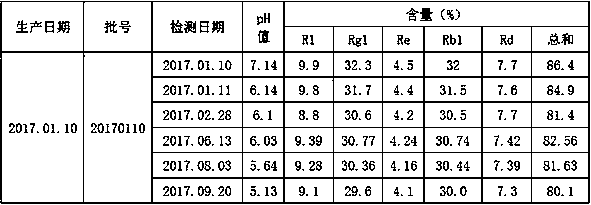
[0078] The product is fully inspected according to the 2015 edition of the Chinese Pharmacopoeia and the quality standard for Xuesaitong injection WS1-B-3590-2001(Z)-2011 and is qualified.
Embodiment 2
[0080] After heating 1500 g of mixed solvent (volume percentage: propylene glycol: polyethylene glycol 400: water for injection = 25%: 25%: 50%) to 55°C, add 25 g of sodium chloride and stir continuously until the dissolution is complete. Add 170g of panax notoginseng saponins and keep stirring until the dissolution is complete. Add 24 g of activated carbon, stir and let it stand for 30 minutes to decarburize to obtain a concentrated medicine. Add 1500 g of mixed solvent (volume percentage: propylene glycol: polyethylene glycol 400: water for injection = 25%: 25%: 50%) to the concentrated medicine, stir and add 6 g of activated carbon, stir and let stand for 30 minutes, decarburize, and filter , Get the liquid medicine. Fill the liquid medicine into the ampoule and seal it. Then sterilize at 100°C for 30 minutes, and perform lamp inspection. Finally, the product is packaged according to the specifications.
[0081] The product is fully inspected according to the 2015 edition o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| relative permittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com