Preparation method and application of solid acid-base stimulus-responsive near-infrared fluorescent compound
A fluorescent compound and stimuli-responsive technology, applied in the field of preparation of near-infrared fluorescent compounds, can solve the problems of small fluorescence shift and low quantum efficiency of solid-state fluorescence, and achieve the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The synthetic method of above-mentioned compound 1, comprises the following steps:
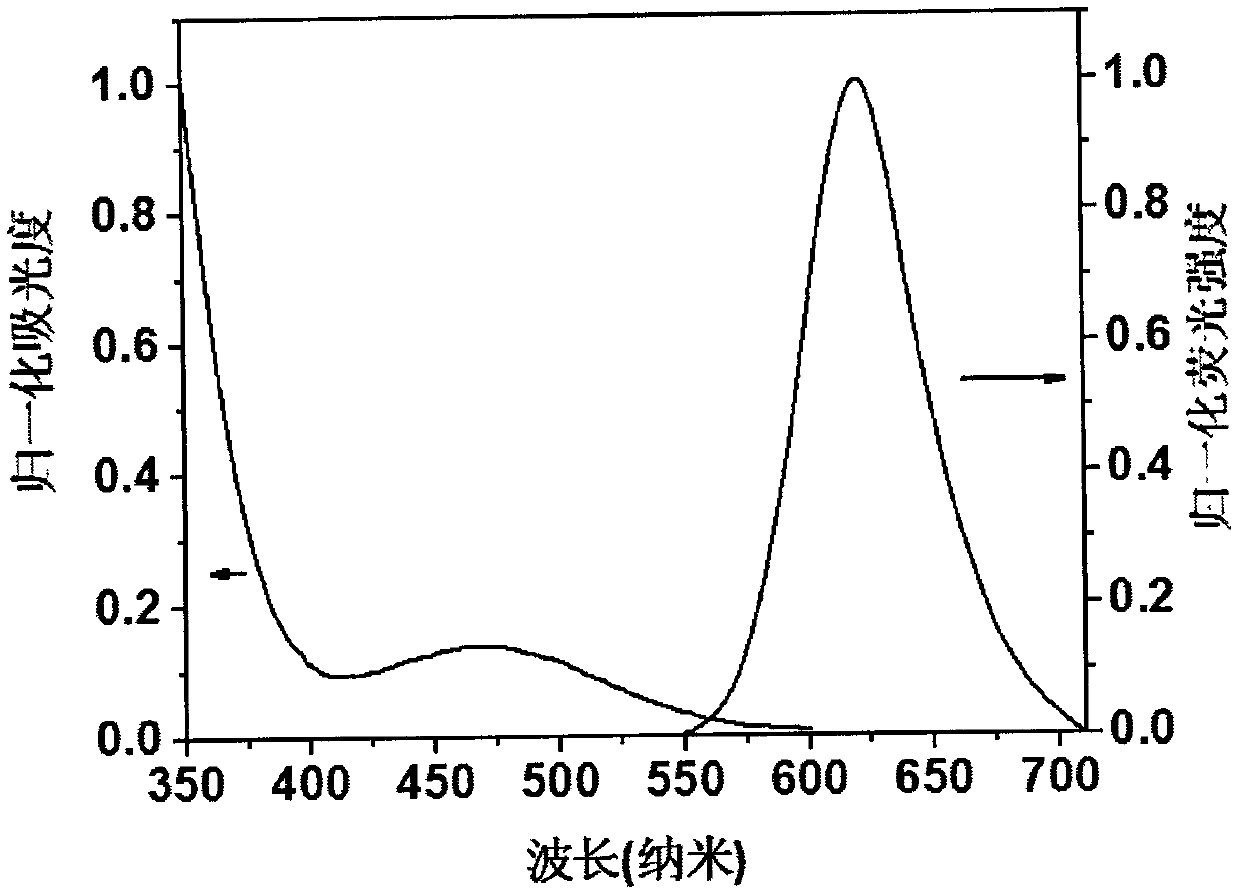
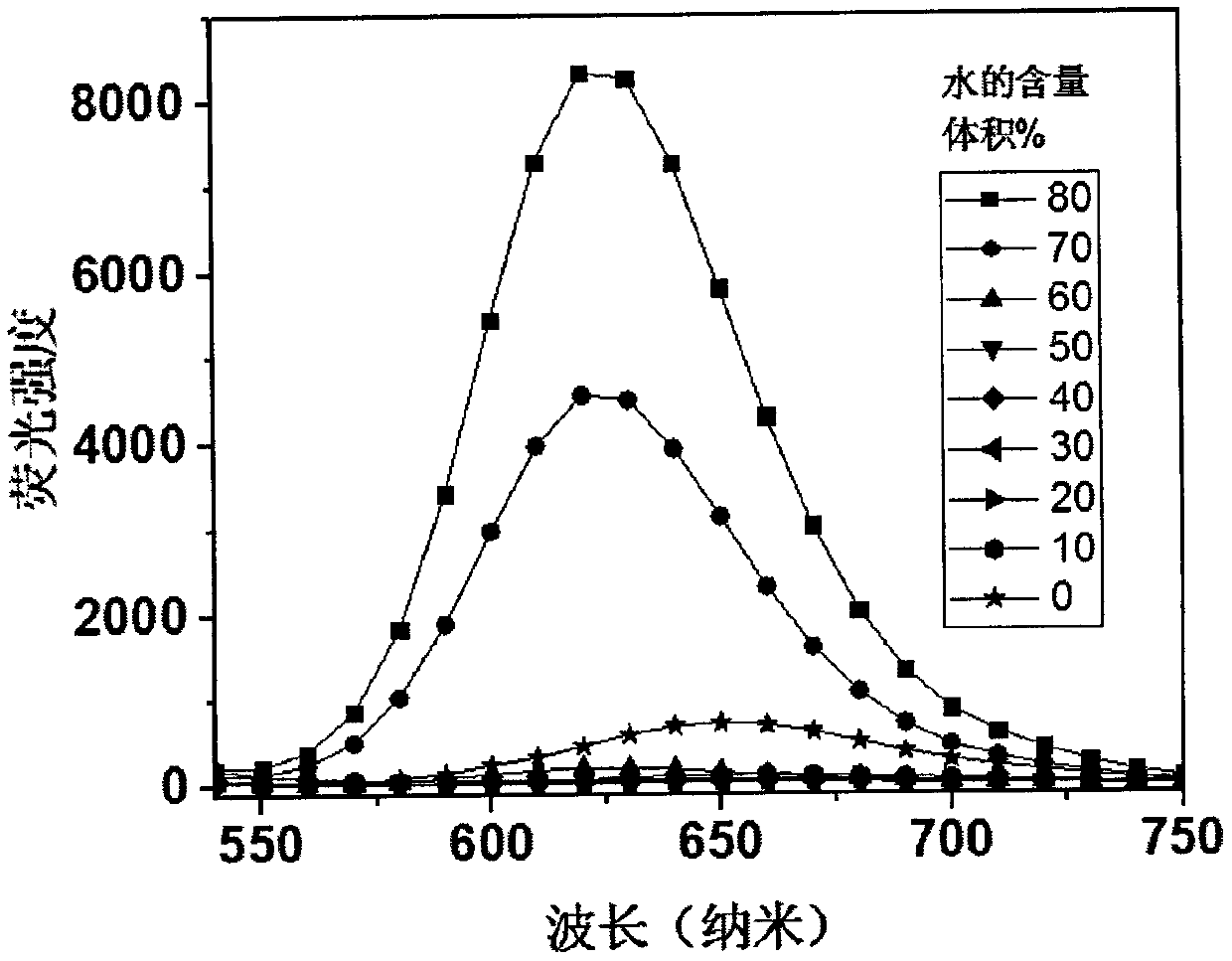
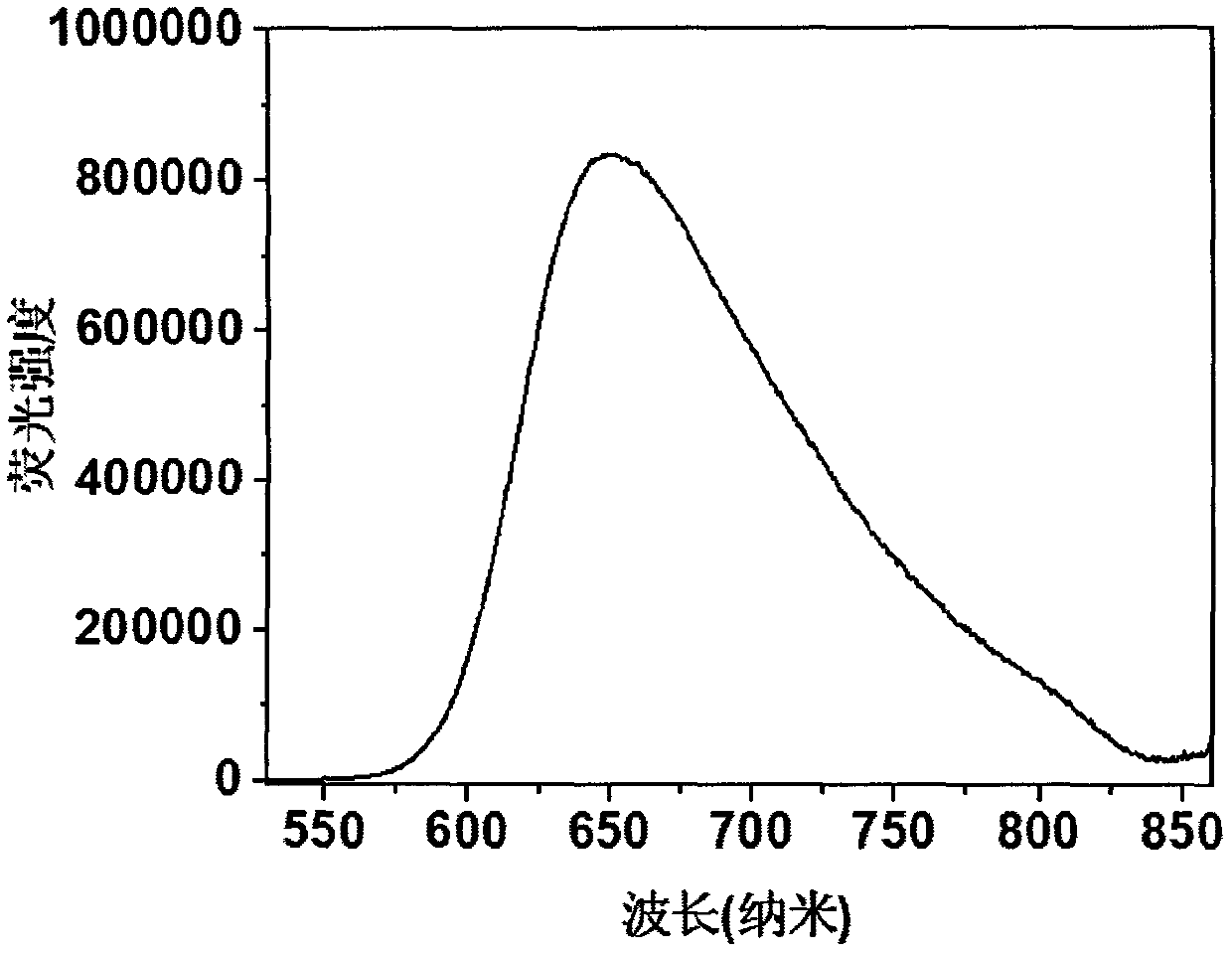
[0024] Compound 1, the molecular formula is C 46 h 32 N 6 , emit red fluorescence under the irradiation of a 365nm ultraviolet lamp, and the molecular chemical formula of compound 1 is as shown in formula (I):
[0025]
[0026] Its preparation method, the steps are as follows:
[0027] 1) In a 250mL round bottom flask, mix 1g (2.13mmol) of 2,5-diphenylamine-1,4-dicarboxybenzene, 504.28mg (4.26mmol) of 3-pyridineacetonitrile and 37.804mL of absolute ethanol mixed to obtain a mixed solution;
[0028] 2) Dissolve 1.32g (33.08mmol) of sodium hydroxide in 37.8mL of absolute ethanol to obtain a sodium hydroxide-ethanol solution;
[0029] 3) Using a constant pressure dropping funnel, add sodium hydroxide-ethanol solution dropwise to the mixed solution under nitrogen protection, stir and react at room temperature for 1 hour, filter after cooling to room temperature, wash the obtained so...
Embodiment 2
[0033] The molecular chemical formula of compound 2 is shown in formula (II): the synthetic method comprises the following steps:
[0034]
[0035] 1) In a 250mL round bottom flask, mix 1g (2.13mmol) of 2,5-diphenylamine-1,4-dicarboxybenzene, 504.28mg (4.26mmol) of 2-pyridineacetonitrile and 37.804mL of absolute ethanol mixed to obtain a mixed solution;
[0036] 2) Dissolve 1.32g (33.08mmol) of sodium hydroxide in 37.8mL of absolute ethanol to obtain a sodium hydroxide-ethanol solution;
[0037] 3) Using a constant pressure dropping funnel, add sodium hydroxide-ethanol solution dropwise to the mixed solution under nitrogen protection, stir and react at room temperature for 1 hour, filter after cooling to room temperature, wash the obtained solid with water and ethanol alternately three times, After drying, it was recrystallized with dichloromethane and ethanol, and filtered to obtain orange-red crystal compound 2, with a yield of 78.2%;
[0038] 1 HNMR (400Hz, CDCl 3 , ...
Embodiment 3
[0040] The molecular chemical formula of compound 3 is shown in formula (III): the synthetic method comprises the following steps:
[0041]
[0042] 1) In a 250mL round bottom flask, mix 1g (2.13mmol) of 2,5-diphenylamine-1,4-dicarboxybenzene, 504.28mg (4.26mmol) of 4-pyridineacetonitrile and 37.804mL of absolute ethanol mixed to obtain a mixed solution;
[0043] 2) Dissolve 1.32g (33.08mmol) of sodium hydroxide in 37.8mL of absolute ethanol to obtain a sodium hydroxide-ethanol solution;
[0044]3) Using a constant pressure dropping funnel, add sodium hydroxide-ethanol solution dropwise to the mixed solution under the protection of nitrogen, stir and react at room temperature for 1 hour, cool to room temperature and filter, and wash the obtained solid with water and ethanol alternately three times, After drying, it was recrystallized with dichloromethane and ethanol, and suction filtered to obtain compound 3 as a purple crystal with a yield of 88.2%;
[0045] 1 HNMR (400...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com