Isoquinoline tricyclic alkaloid compound as well as preparation method and application thereof
A technology of alkaloids and compounds, applied in the fields of botanical equipment and methods, chemicals for biological control, applications, etc., to achieve the effects of large biological yield, high alkaloid content and wide distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
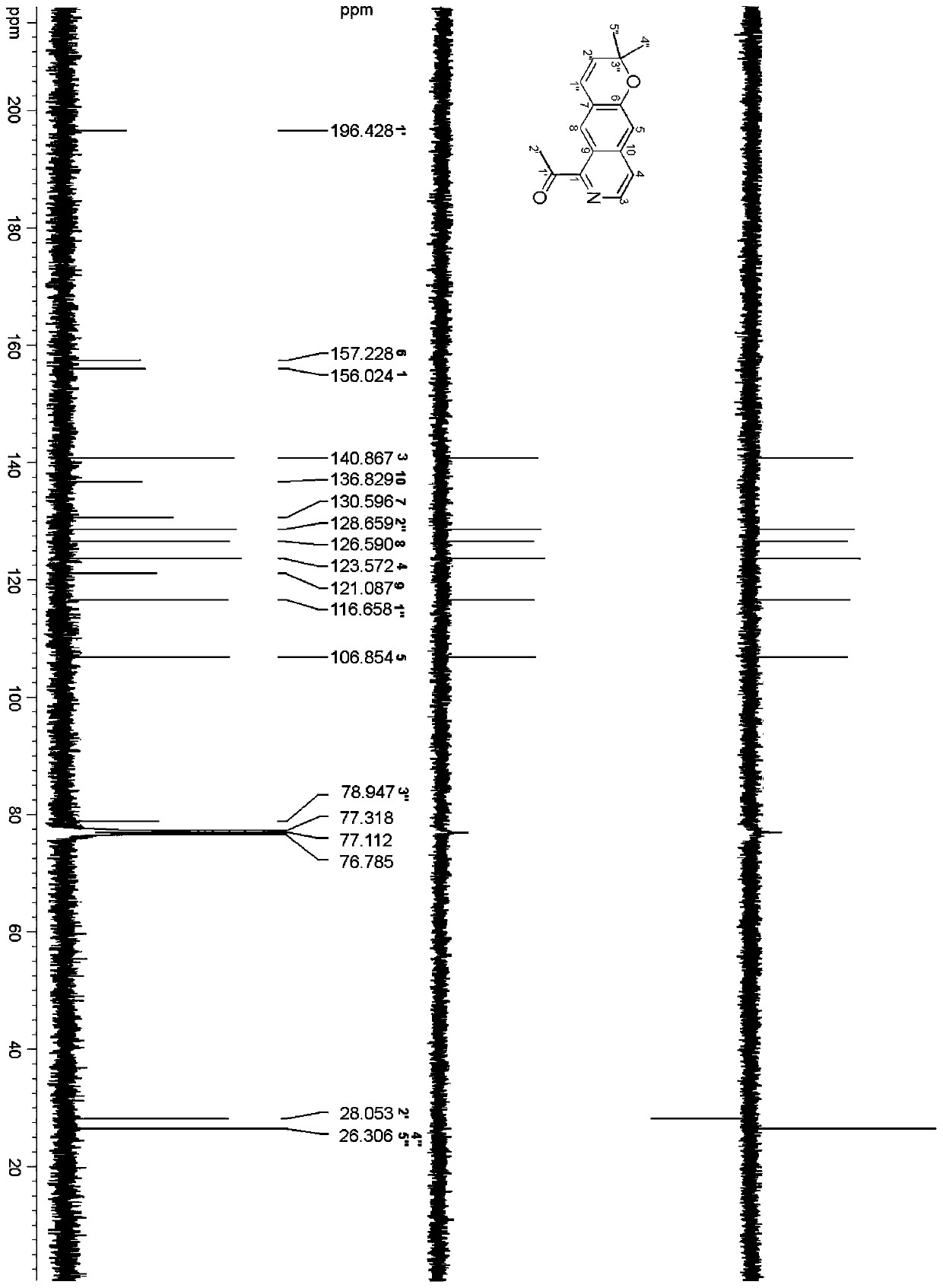
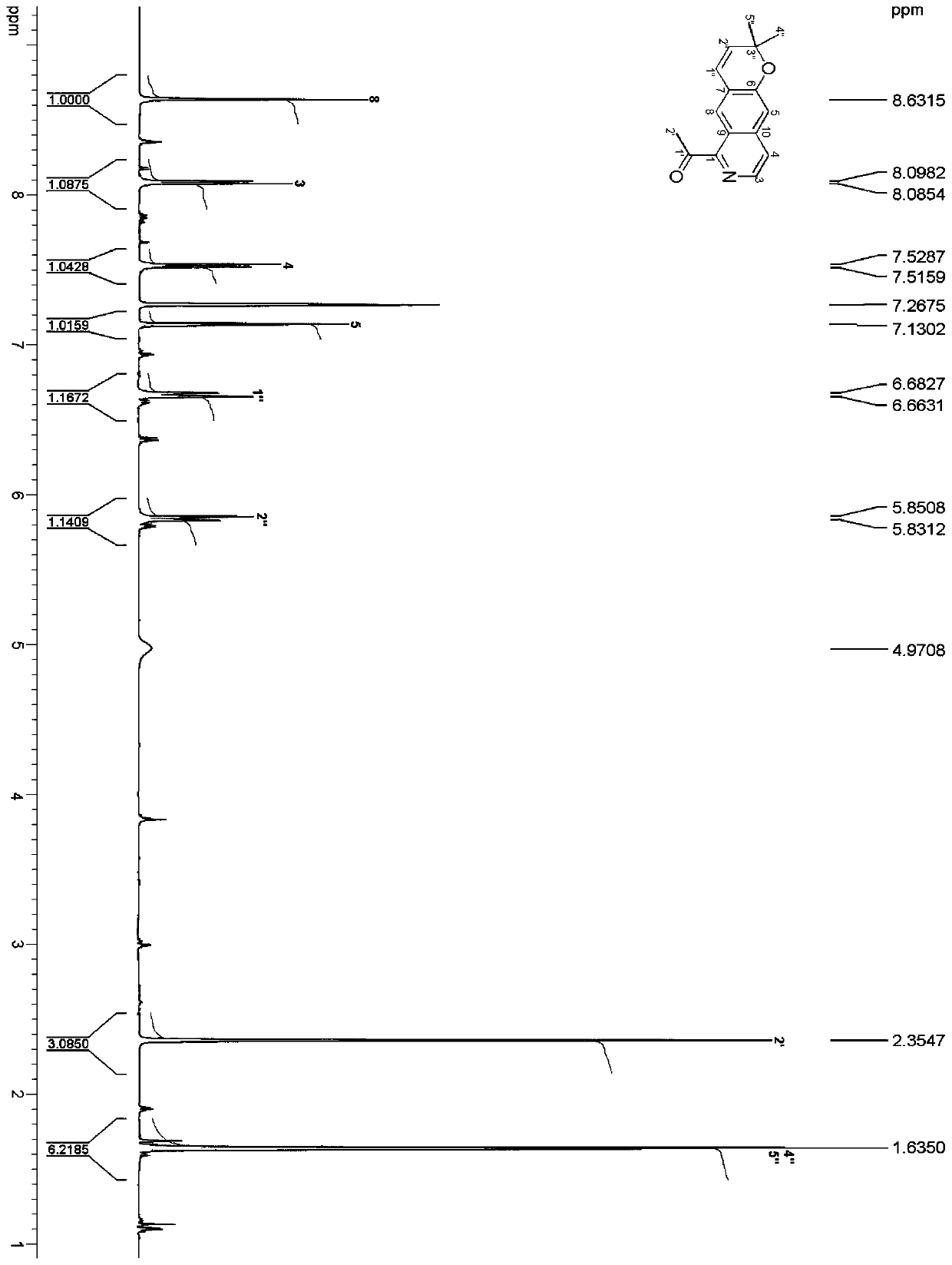
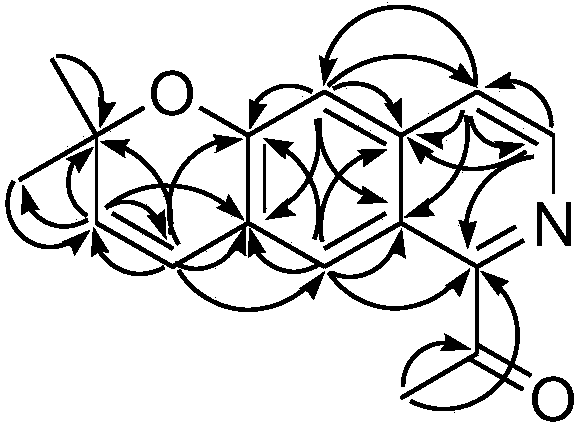
[0038] Preparation of isoquinoline tricyclic alkaloid compound C 16 h 15 NO 2 , including extracting extract, silica gel column chromatography, and high-pressure liquid chromatography separation, specifically adopting the following steps:
[0039] (1) Extraction of extract: take the whole plant of Sargassum aureus, dry it in the sun, and crush it to 30-50 mesh. Weigh 3.0-4.5kg of the pulverized sample, put it in a 20L glass reaction kettle, add 10-15L of 95% ethanol, reflux for 30-50min, and filter out the extract; add 95% ethanol 10L to the filter residue again ~15L, reflux extraction for 30~50min, filter the extract. The two extracts were combined and concentrated to a small volume, then diluted with 5-8L of 3% tartaric acid solution, and extracted twice with 5-8L of ethyl acetate. After the extraction, the aqueous phase was saturated with sodium carbonate, extracted twice again with 5-8 L of ethyl acetate, the ethyl acetate phase extracted for the second time was combin...
Embodiment 2
[0044] The sample of horsetail lotus came from Heqing County, Dali, Yunnan. Get the whole plant of Saccharomyces japonicus and dry it in the sun, and crush it to 35 meshes. Weigh 3.0kg of the pulverized sample, place it in a 20L glass reactor, add 12L of 95% ethanol, reflux for extraction for 40min, and filter the extract; add 12L of 95% ethanol to the filter residue again, reflux for 40min, filter Remove the extract. The two extracts were combined and concentrated to a small volume, then diluted with 6L of 3% tartaric acid solution, and extracted twice with 6L of ethyl acetate. After the extraction, the aqueous phase was saturated with sodium carbonate, extracted twice with 6 L of ethyl acetate, and the extracted ethyl acetate phases were combined and concentrated under reduced pressure to obtain 48.2 g of alkaloid part extract. The extract is mixed with 60g (80-100 mesh) thick silica gel, dried, and 180g silica gel (150-200 mesh) column chromatography, chloroform: acetone ...
Embodiment 3
[0046] The samples of horsetail lotus came from Binchuan County, Dali, Yunnan. Get the whole plant of Saccharomyces japonicus and dry it in the sun, and crush it to 40 mesh. Weigh 3.8kg of crushed sample, put it in a 20L glass reaction kettle, add 14L of 95% ethanol, reflux for extraction for 30min, and filter out the extract; add 14L of 95% ethanol to the filter residue again, reflux for 30min, filter Remove the extract. The two extracts were combined and concentrated to a small volume, then diluted with 8 L of 3% tartaric acid solution, and extracted twice with 8 L of ethyl acetate. After the extraction, the aqueous phase was saturated with sodium carbonate, extracted twice again with 8 L of ethyl acetate, and the extracted ethyl acetate phases were combined and concentrated under reduced pressure to obtain 46.7 g of alkaloid part extract. The extract is mixed with 60g (80-100 mesh) thick silica gel, dried, and 220g silica gel (150-200 mesh) column chromatography, chlorofo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com