Synthesis method of ibrutinib intermediate
A technology of ibrutinib and intermediates, applied in the field of medicine, can solve the problems of harsh synthesis conditions, unsuitable for industrial production, expensive and the like, and achieve the effects of short synthesis steps and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
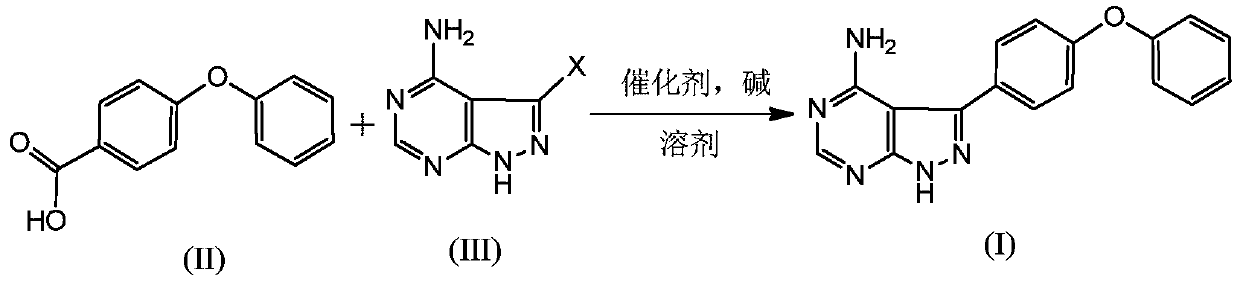
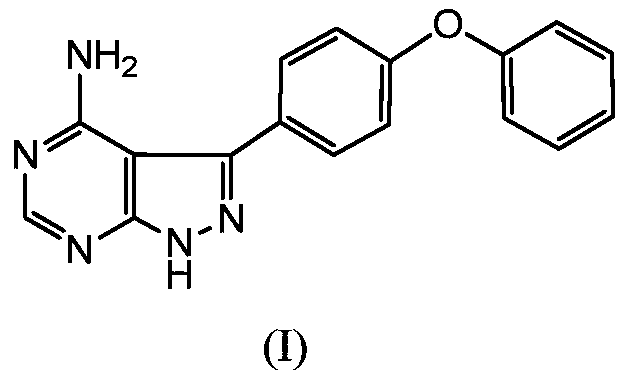
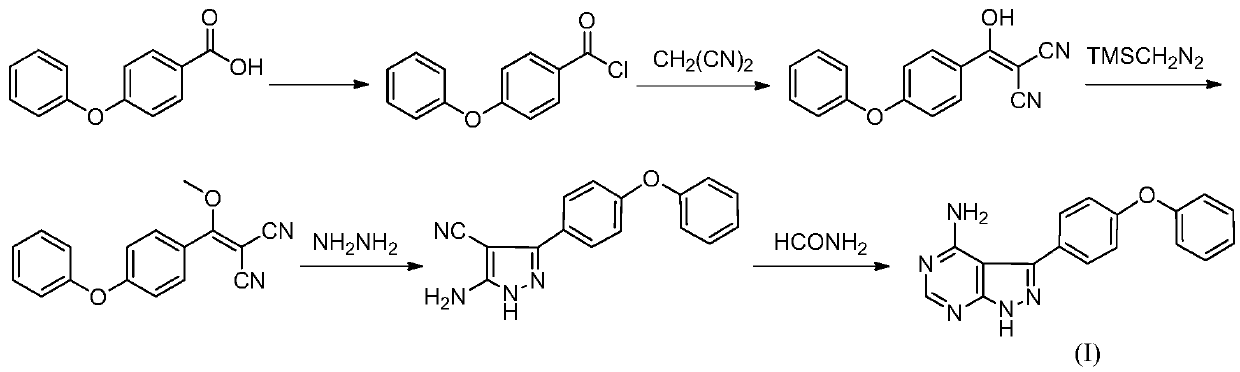
[0035] Add N,N-dimethylformamide (50mL) to the reaction flask, add 4-phenoxybenzoic acid (II) (4.37g, 0.02mol) under nitrogen protection, 3-chloro-4-amino-1H-pyridine Azolo[3,4,d]pyrimidine(III) (5.19g, 0.03mol), potassium phosphate (17.14g, 0.08mol), CuI (0.38g, 0.002mol) and pyridine (0.65g, 0.008mol). The temperature was raised to 150° C. for reflux reaction for 12 hours, and the reaction was detected by HPLC to be complete (the content of 4-phenoxybenzoic acid was less than 1%). Cool down to room temperature, slowly add water to precipitate a solid, filter, and dry to obtain a solid product (3.89 g, yield: 64.1%).
Embodiment 2
[0037] N,N-dimethylacetamide (50mL) was added to the reaction flask, and 4-phenoxybenzoic acid (II) (4.81g, 0.022mol), 3-bromo-4-amino-1H- Pyrazolo[3,4,d]pyrimidine (III) (4.37g, 0.02mol), sodium carbonate (8.74g, 0.1mol), CuBr (0.43g, 0.003mol), 1,10-phenanthroline (0.73 g, 0.004mol). The temperature was raised to 150°C for 24h. Cool down to room temperature, slowly add water to precipitate a solid, filter, and dry to obtain a solid product (4.95 g, yield: 81.5%).
Embodiment 3
[0039] Dimethylsulfoxide (50mL) was added into the there-necked flask, and 4-phenoxybenzoic acid (II) (8.74g, 0.04mol) was added under nitrogen protection, 3-iodo-4-amino-1H-pyrazolo[ 3,4,d] pyrimidine(III) (5.33g, 0.02mol), cesium carbonate (7.87g, 0.04mol), CuOAc (0.49g, 0.004mol) and 2,2'-bipyridine (0.32g, 0.002mol ). The temperature was raised to 140°C for 10 hours. Cool down to room temperature, slowly add water to precipitate a solid, filter, and dry to obtain a solid product (5.08 g, yield: 83.7%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com