Method for micronization of raw materials of dihydropyridine antihypertensive drugs
A technology of drug addition, applied in the field of micronization of dihydropyridine antihypertensive drug raw materials, which can solve the problems of difficult particle size, low yield, and high energy consumption in the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
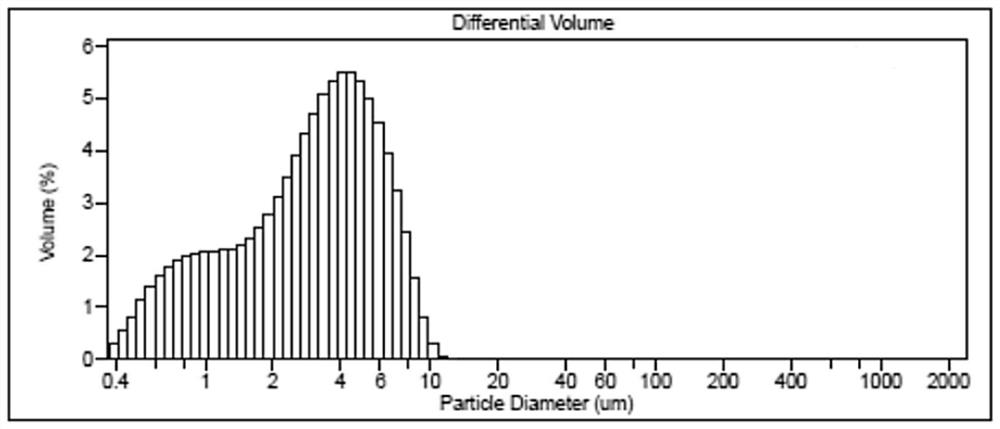
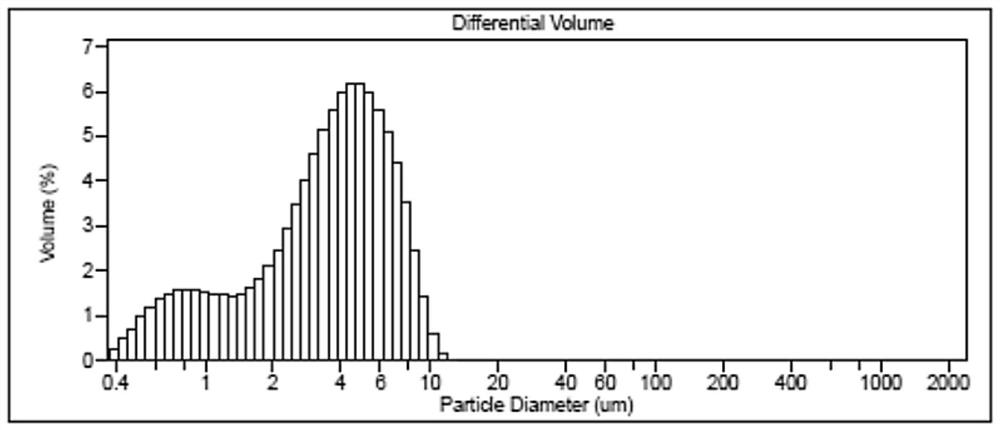
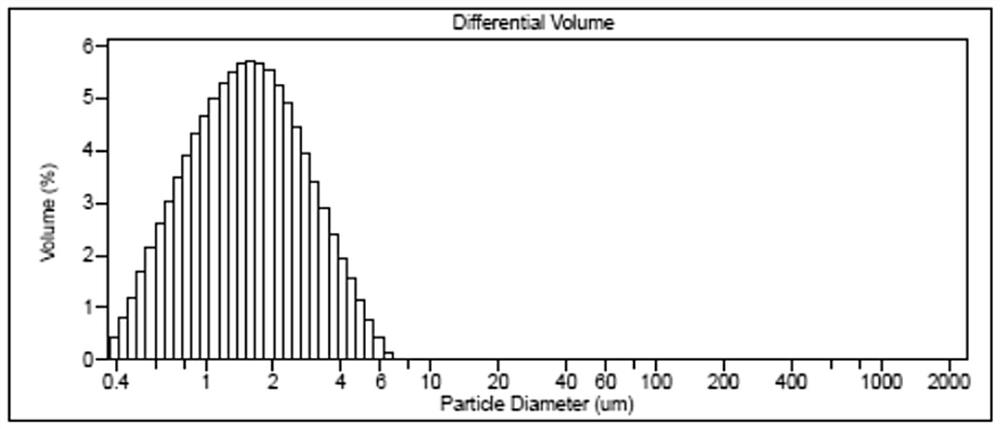
Image
Examples
Embodiment 1
[0069] Get and meet " Chinese Pharmacopoeia " 2015 edition quality standards Felodipine crude drug powder (D90 is 76 μ m) 10.0g, be placed in the 50ml round bottom flask that is equipped with condenser, add concentration and be 95% ethanol (the first solvent) 30.0g mL, and then under electromagnetic stirring, dissociate in a 60°C water bath until completely dissolved, then continue to heat and stir for 5 minutes, then remove the hot water bath, and let the solution naturally cool to room temperature for later use.
[0070] Pour the prepared ethanol (first solvent) solution in which the felodipine crude drug powder is dissolved into a preheated dropping funnel for subsequent use.
[0071] A 1500ml reaction flask equipped with an electric stirrer and a thermometer was placed in an ice-salt water pot, and then 900mL of purified water (second solvent) was added to the round bottom flask. After the addition was complete, the electric stirring was started to cool down.
[0072]When ...
Embodiment 2~ Embodiment 22
[0076] The main process parameters of Examples 2-22 are recorded in Table 1, and for other parameters not recorded in Table 1, please refer to the content of Example 1. Wherein, the second solvent of embodiment 2~20 is water, and the second solvent of embodiment 21 is the sodium chloride aqueous solution that concentration is 1.0%, and the second solvent of embodiment 22 is the lauryl sulfuric acid of concentration 0.4% sodium solution.
Embodiment 23
[0078] Get the felodipine crude drug powder (D 90 76μm) 10g, put in a round bottom flask, then add 30mL of prepared ethanol (the first solvent), and heat it on a 60°C water bath until it is completely dissolved, then continue to keep stirring for 5min, then remove the hot water bath, and dissolve the solution Allow to cool naturally to room temperature for later use.
[0079] Pour the prepared ethanol solution dissolving the felodipine crude drug powder into the preheated spray gun for standby.
[0080] A 1500mL round-bottomed flask equipped with an electric stirrer and a thermometer was placed in an ice-salt bath, and then 900mL of purified water (second solvent) was added to the round-bottomed flask. After the addition was complete, the electric stirring was started to cool down.
[0081] When the water temperature in the round bottom flask drops to about 1°C, adjust the stirring speed to 650r / min, then adjust the spray gun pressure to 35KPa, and spray the above concentrati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com