Method for preparing Ti-doped and La4NiLiO8-coated nickel-rich positive electrode material
A positive electrode material, nickel-rich technology, applied in the direction of positive electrode, battery electrode, active material electrode, etc., can solve the problem of reducing electrochemical performance, achieve the effect of promoting electron transfer, inhibiting side reactions, and reducing heat generation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
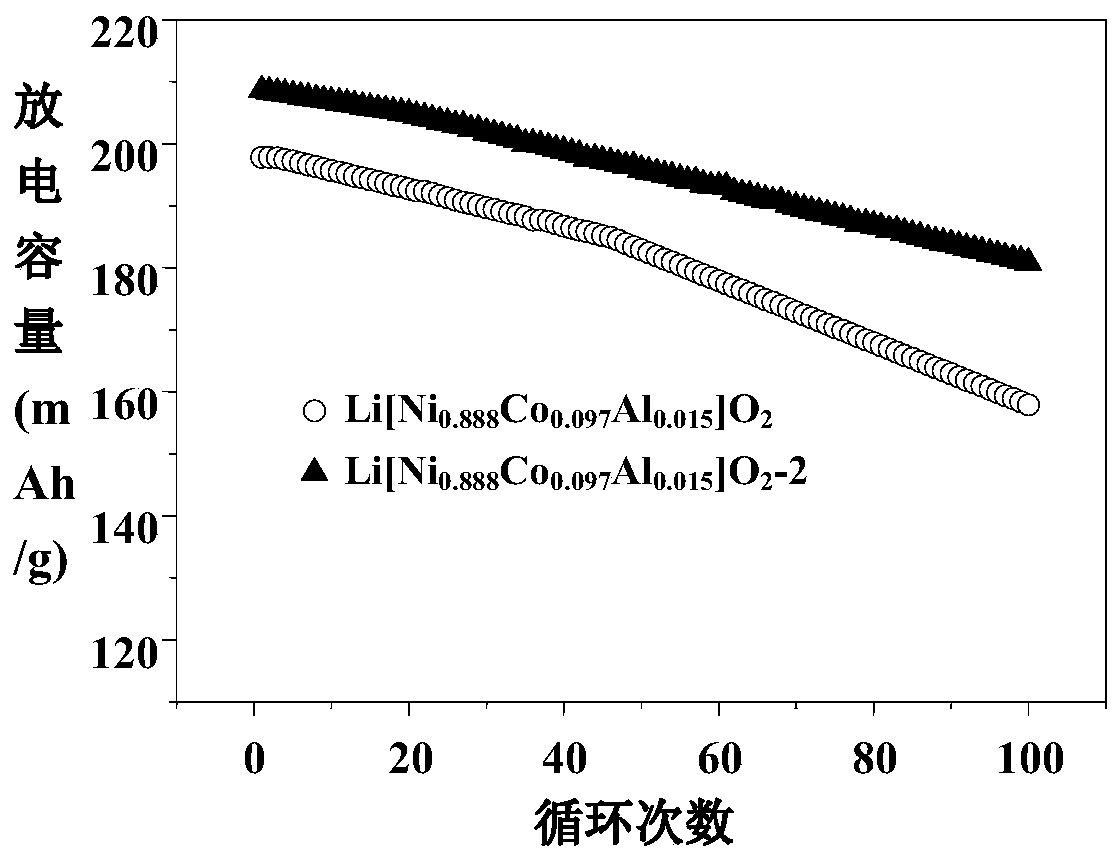
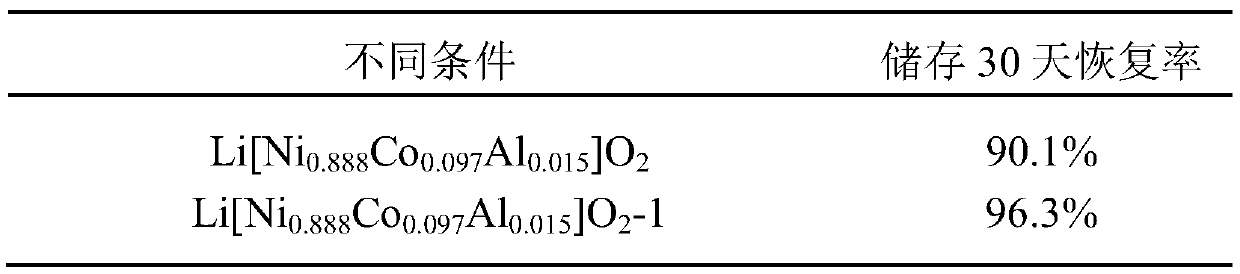
[0016] Ti doping and La 4 NiLiO 8 Coated Li[Ni 0.888 co 0.097 Al 0.015 ]O 2 Preparation of nickel-rich material: 257.74g of NiSO 4 ·6H 2 O and 5.62 g of CoSO 4 ·7H 2 O was dissolved in deionized water; 76 g of NaOH solution and 38.5 g of ammonia water were added to the reactor, and then NiSO 4 and CoSO 4 Solution is added in the reactor under the protection of nitrogen, obtains [Ni 0.98 co 0.02 ](OH) 2 Precursor.
[0017] Dissolve 102g of tetrabutyl titanate and 31.6g of lanthanum acetate in 60mL of ethanol, place the solution in a 60°C water bath and stir to dissolve completely, and dissolve 570g of [Ni 0.98 co 0.02 ](OH) 2 , 393.38 g of LiOH·H 2 O, 11.57g of Al(OH) 3 ·3H 2 O solution, add tetrabutyl titanate and lanthanum acetate mixed liquid and stir, then filter the solution, dry, and calcined at 700° C. for 12 hours. Finally get Li[Ni 0.888 co 0.097 Al 0.015 ]O 2 -1.
Embodiment 2
[0019] Ti doping and La 4 NiLiO 8 Coated Li[Ni 0.888 co 0.097 Al 0.015 ]O 2 Preparation of nickel-rich material: 257.74g of NiSO 4 ·6H 2 O and 5.62 g of CoSO 4 ·7H 2 O was dissolved in deionized water; 76g of NaOH solution and 42g of ammonia water were added to the reactor, and then NiSO 4 and CoSO 4 Solution is added in the reactor under the protection of nitrogen, obtains [Ni 0.98 co 0.02 ](OH) 2 Precursor,
[0020] Dissolve 160g of tetrabutyl titanate and 54g of lanthanum acetate in 60mL of ethanol, place the solution in a water bath at 60°C and stir to dissolve it completely, and dissolve 753g of [Ni 0.98 co 0.02 ](OH) 2 , 371.7g of LiOH·H 2 O, 18g of Al(OH) 3 ·3H 2 O solution, add tetrabutyl titanate and lanthanum acetate mixed solution and stir, then filter, dry and calcinate the solution at 730° C. for 10 h. Finally get Li[Ni 0.888 co 0.097 Al 0.015 ]O 2 -2.
Embodiment 3
[0022] Ti doping and La 4 NiLiO 8 Coated Li[Ni 0.888 co 0.097 Al 0.015 ]O 2 Preparation of nickel-rich material: 257.74g of NiSO 4 ·6H 2 O and 5.62 g of CoSO 4 ·7H 2 O was dissolved in deionized water; 80 g of NaOH solution and 42 g of ammonia water were added to the reactor, and then NiSO 4 and CoSO 4 Solution is added in the reactor under the protection of nitrogen, obtains [Ni 0.98 co 0.02 ](OH) 2 Precursor.
[0023] Dissolve 238g of tetrabutyl titanate and 150g of lanthanum acetate in 60mL of ethanol, place the solution in a water bath at 60°C and stir to dissolve it completely, and dissolve 729.52g of [Ni 0.98 co 0.02 ](OH) 2 , 375.57 g of LiOH·H 2O, 23.14g of Al(OH) 3 ·3H 2 O solution, add tetrabutyl titanate and lanthanum acetate mixed solution and stir, then filter the solution, dry, and calcined at 750° C. for 8 hours. Finally get Li[Ni 0.888 co 0.097 Al 0.015 ]O 2 -3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com