Use of pdf inhibitor for preparing medicine for treating intestinal inflammatory diseases
A PDGF-C, inhibitor technology, applied in the field of biomedicine, can solve the complex mechanism of radiation enteropathy and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
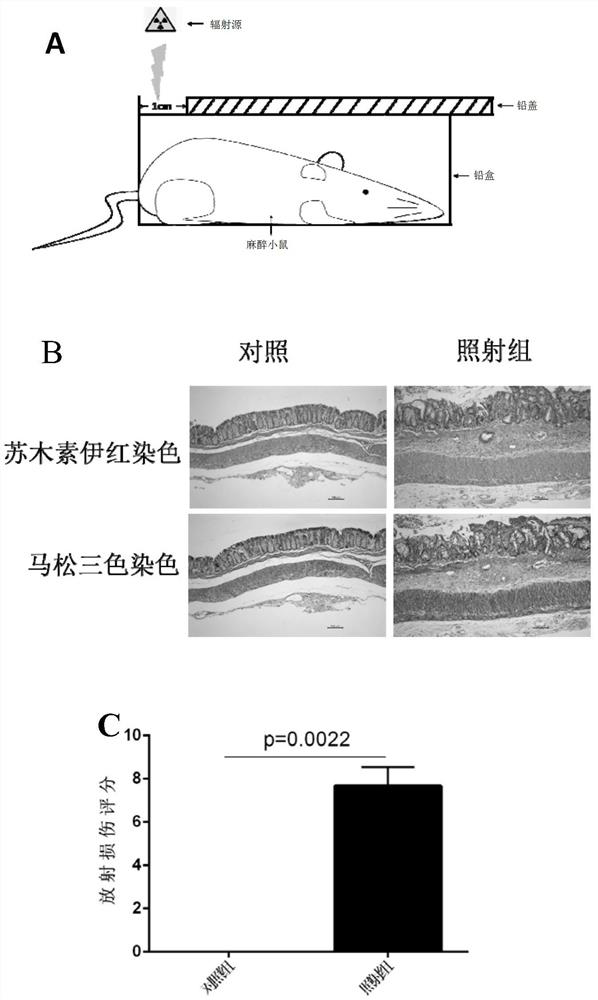
[0147] Embodiment 1 Animal experiment
[0148] 1.1 Establishment of animal models of radiation enteropathy
[0149] According to existing literature reports, 6-8 week old female C57BL6 / J mice were used as modeling objects. The experimental procedures and animal handling methods complied with the animal experiment guidelines and rules of the Experimental Animal Ethics and Welfare Committee of Sun Yat-sen University.
[0150] (1) Purchase 8-week-old female C57BL6 / J mice from the Animal Experiment Center of Sun Yat-sen University, weighing about 18g-20g, place them in the Animal Experiment Center of the North Campus of Sun Yat-sen University for one week and then irradiate them.
[0151] (2) The mice were ear-marked and numbered one day before irradiation, and the mice were divided into the irradiation group and the control group by using the Excel table to generate random numbers. At the same time, the mice in the two groups were weighed and recorded.
[0152] (3) On the day o...
Embodiment 2
[0171] Embodiment 2 cell culture and processing
[0172] (1) CCD-18Co (human colon fibroblast) cell culture
[0173] Place the CCD-18Co cells preserved in liquid nitrogen in a water bath at 37°C for 3-5 minutes, then resuspend them in DMEM medium containing 10% fetal bovine serum, and store them in 37%, 5% CO 2 Cultured under the condition of 1:3~1:5 when the cells grew to 90% monolayer.
[0174] (2) Crenolanib treatment of CCD-18Co cells
[0175] When the cells grow to 80%-90% monolayer, absorb the culture solution containing 10% fetal bovine serum in the cell culture dish to be treated, add an appropriate amount of serum-free DMEM culture solution to starve overnight, and then add the prepared Crenolainib (CP- 868596) solution (5mM / ml) was added to the DMEM culture solution containing 10% fetal bovine serum, the treatment concentration was adjusted to 1 μM / ml, and the cell protein was collected after continuing to culture for 48 hours.
[0176] (3) Anti-PDGFRα or anti-PDG...
Embodiment 3
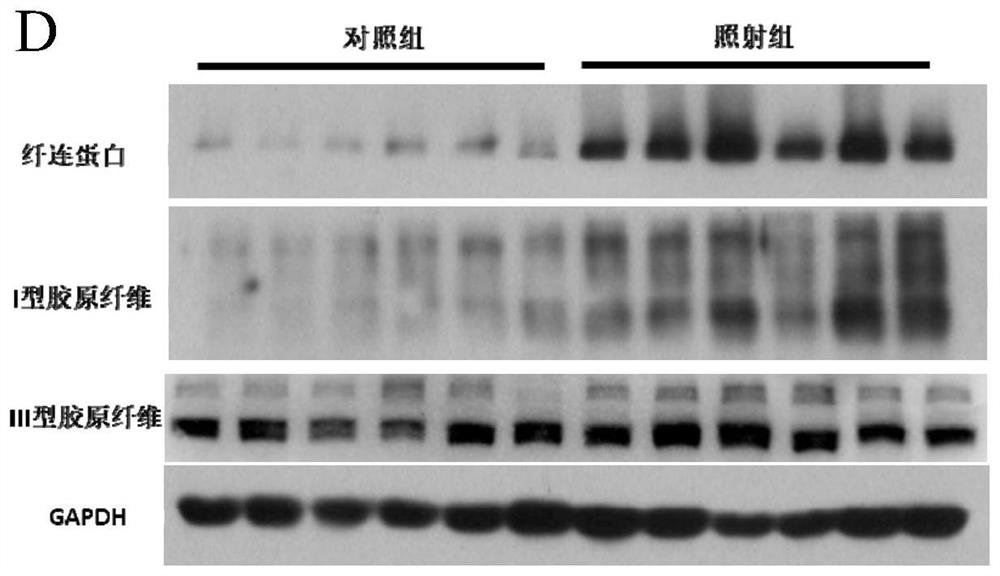
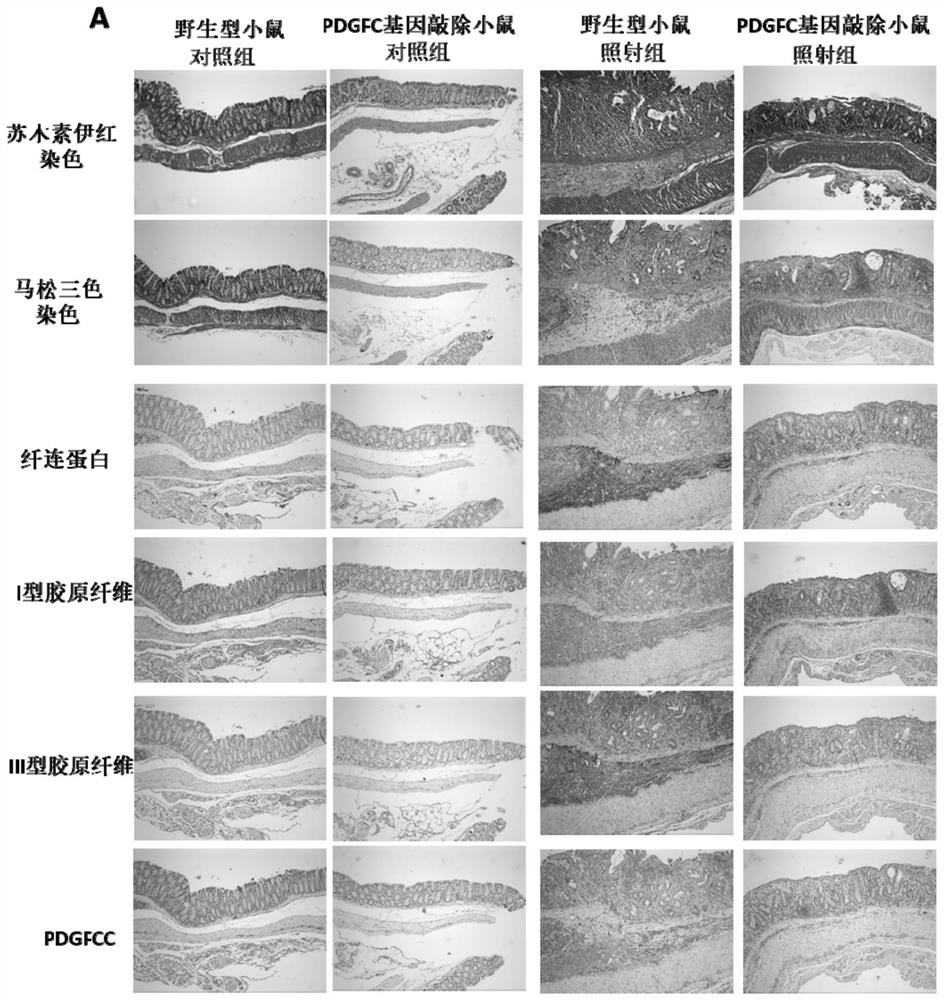
[0187] HE, MASSON, immunohistochemical staining and RIS score of embodiment 3 animal tissue
[0188] (1) HE staining
[0189] The mouse intestinal tissue used for HE (hematoxylin-eosin staining) staining was placed in 10% neutral formalin fixative solution for 24 hours, rinsed with running water, dehydrated, transparent, and embedded in paraffin to prepare a 4 μm thick Serial paraffin sections. Paraffin sections were routinely dewaxed to water, stained with hematoxylin at room temperature for 10 minutes, rinsed with tap water for 30-60 seconds; differentiated with 1% hydrochloric acid alcohol for 1 second, rinsed with tap water for 1 minute; stained with eosin at room temperature for 5-10 minutes; dehydrated with gradient alcohol; transparent with xylene; neutral gum cover film.
[0190] (2) MASSON staining
[0191] The intestinal tissues of mice used for MASSON staining were placed in 10% neutral formalin fixative solution for 24 hours, rinsed with running water, dehydrate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com