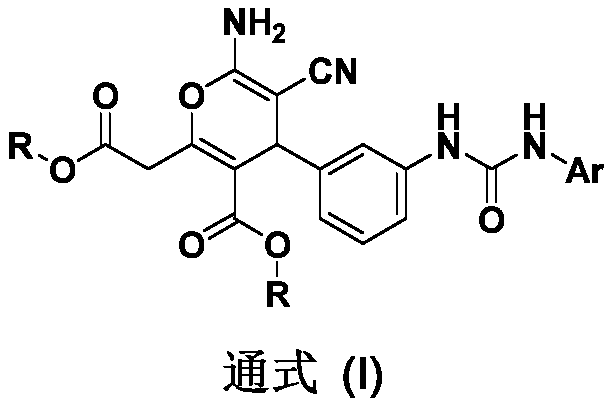
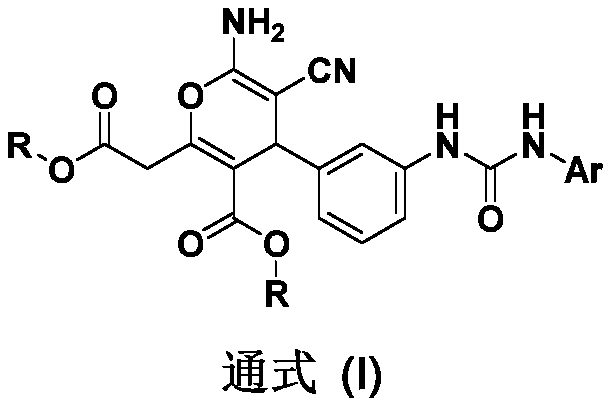
Novel arylurea-containing 4-arylpyran derivative and application thereof
A technology of arylpyrans and aryl urea, which is applied in the field of medicine, can solve the problems of poor compound activity, poor druggability, structural modification, etc., and achieve the effect of preventing colon cancer and lung cancer and having significant anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Compound 1 6-amino-5-cyano-2-(2-methoxy-2-oxoethyl)-4-[3-(3-phenylureido)phenyl)-4H- Methyl pyran-3-carboxylate
[0047]
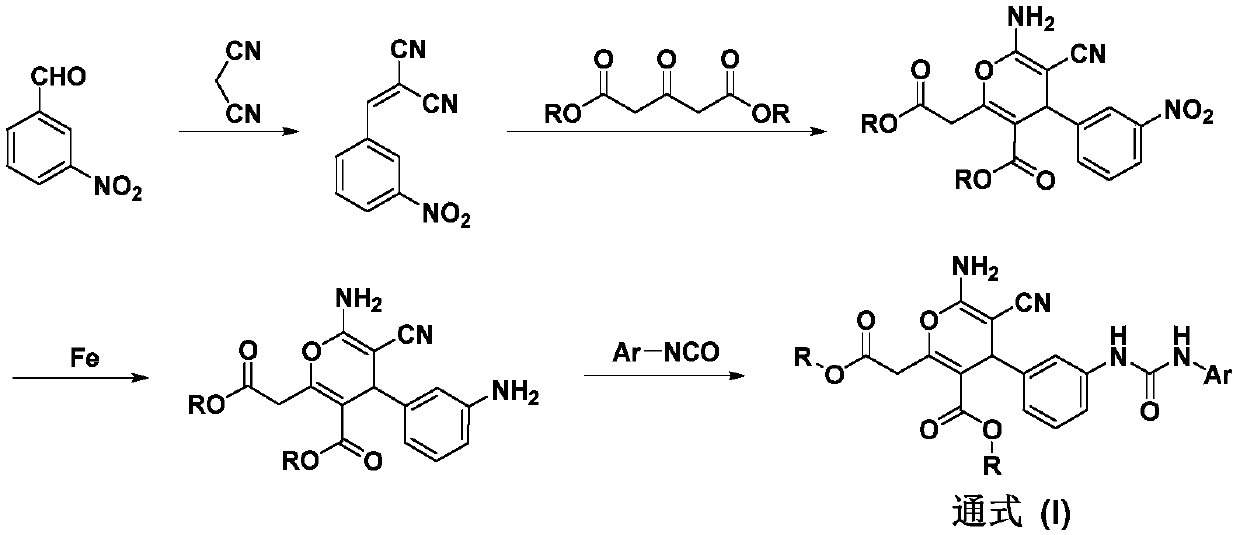
[0048] Preparation of step A 2-(3-nitrobenzylidene) malononitrile
[0049]
[0050] Take a 250mL reaction flask, add 3-nitrobenzaldehyde (15.00g, 99.3mmol), dissolve it with 100mL ethanol, add malononitrile (7.20g, 109.2mmol) in 60mL ethanol solution to the above dissolution, add triacetate Amine 2.0mL, stirred at room temperature for 2h, a large amount of solids precipitated during the reaction, filtered, the filter cake was washed three times with cold ethanol, and dried to obtain 17.6g of yellow solid, namely 2-(3-nitrobenzylidene) propane Nitrile.
[0051] Step B of 6-amino-5-cyano-2-(2-methoxy-2-oxoethyl)-4-(3-nitrophenyl)-4H-pyran-3-carboxylic acid methyl ester preparation
[0052]
[0053] Get a 250mL reaction bottle, add 2-(3-nitrobenzylidene) malononitrile (13.00g, 65.3mmol) respectively, dimethyl acetone dicarboxyla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com