Pregabalin capsule and preparation method thereof
A pregabalin and capsule technology, applied in the field of medicine, can solve the problems of poor compressibility of pregabalin, difficulty in forming granules to improve fluidity, etc., and achieve the effects of stable dissolution behavior without change and improving dissolution rate.
Inactive Publication Date: 2019-12-27
HANGZHOU BIO SINCERITY PHARMA TECH CO LTD
View PDF2 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Therefore, wet granulation (especially wet granulation with lactose as filler) is not suitable for the preparation of pregabalin capsules
[0011] (2) The compressibility of pregabalin is extremely poor, so it is difficult to form granules and improve fluidity by dry granulation
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1~3
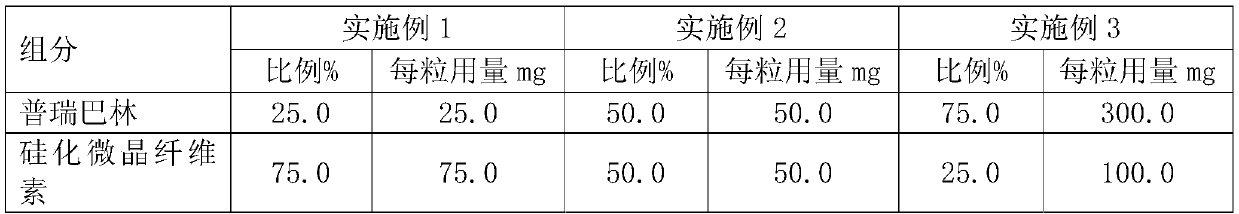
[0020] Table 1: Prescription Table of Embodiments 1 to 3
[0021]
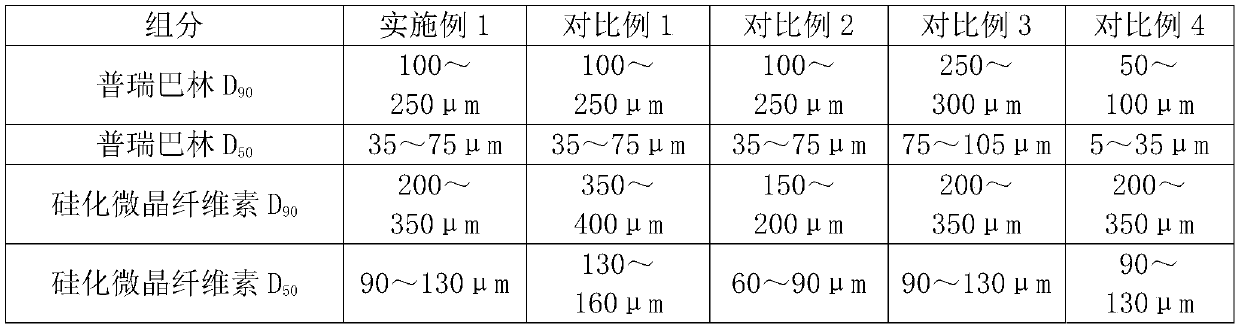
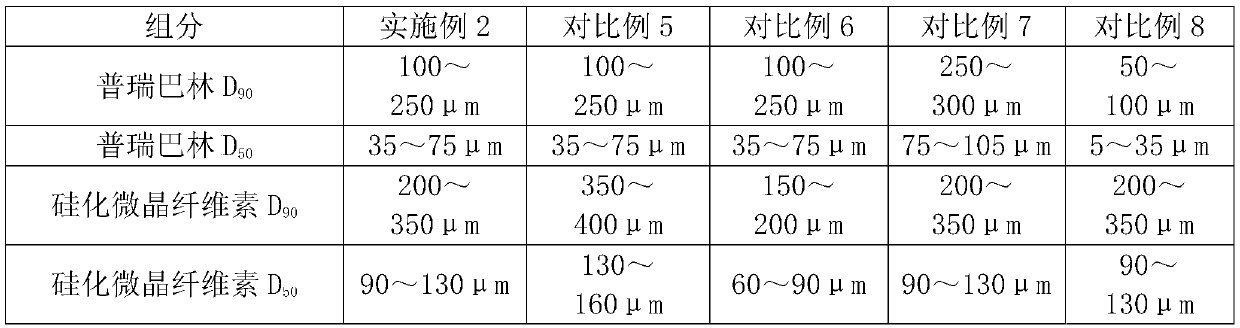
[0022] Choose pregabalin particle size distribution: D 90 100~250μm, D 50 35~75μm; choose the particle size distribution of silicified microcrystalline cellulose: D 90 200-350 μm; D 50 It is 90-130 μm. The above unit dose composition is placed in a gelatin hollow capsule.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses a pregabalin capsule and a preparation method thereof. The content is composed of pregabalin and silicified microcrystalline cellulose, wherein the silicified microcrystallinecellulose is prepared by blending microcrystalline cellulose and micro-powder silica gel in water and performing drying. In the pregabalin capsule, a weight percentage of pregabalin is 25-75%, D90 is100-250 [mu]m, and D50 is 35-75 [mu]m; and D90 of the excipient silicified microcrystalline cellulose is 200-350 [mu]m, and D50 is 90-130 [mu]m. The production process is simple, the product is stable, and the pregabalin capsule is suitable for large-scale industrial production.
Description
technical field [0001] The invention belongs to the technical field of medicine, and in particular relates to a pregabalin capsule and a preparation method thereof. Background technique [0002] Pregabalin is a new type of antiepileptic drug. Its molecular structure has γ-aminobutyric acid structure, so it has anticonvulsant effect. [0003] Pregabalin is a class I drug in the BCS classification system. Pregabalin reaches its peak concentration after 1.5 hours of oral administration, and its relative bioavailability is ≥90%, and C max And AUC has a linear relationship with the dose, it is not combined with plasma protein in vivo, and has almost no metabolism. [0004] Pregabalin was successfully developed by Pfizer Europe MA EEIG. It was first approved for marketing by the European Medicines Agency (EMA) on July 6, 2004. 100mg, 150mg, 200mg, 225mg, 300mg), oral liquid (specification: 20mg / ml). [0005] The EMA review report shows that the pregabalin capsules (trade name ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K9/48A61K31/197A61K47/38A61P25/08
CPCA61K31/197A61K9/4866A61P25/08
Inventor 陈晓萍李柳洋邹永华陆勤霞巴文静潘晨
Owner HANGZHOU BIO SINCERITY PHARMA TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com