Cell necrosis inhibitor, and preparation method and application thereof
A compound, unsubstituted technology, used in anti-inflammatory agents, antibacterial drugs, pharmaceutical formulations, etc., can solve problems such as limited research and clinical applications, inability to enter the central nervous system through the blood-brain barrier, and poor pharmacokinetic properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
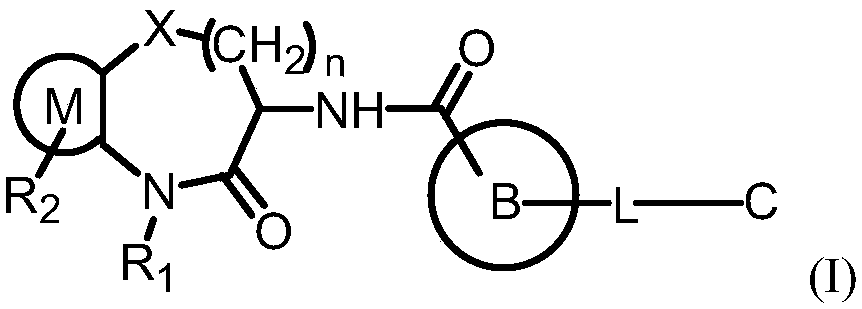
[0148] The preparation of formula I compound
[0149] 1. The compound of formula I can be prepared by the following methods:
[0150] 1) Amide condensation: Fragment II first removes the protective agent on the amino group under acidic conditions, and then condenses with Fragment III to obtain the target compound
[0151]
[0152] Some examples are as follows:
[0153]
[0154] (a) in an inert solvent, in the presence of a condensation reagent and a base, react with a compound of formula II and a compound of formula III to obtain a compound of formula I; wherein R is H;
[0155] Wherein, solvent can be: DMF, DMSO, acetonitrile, THF, DCM or its combination
[0156] The condensation reagent can be: HATU, DCC, HOBt, HBTU, HCTU, TBTU, TSTU, TNTU, EDCI, CDI, PyBOP or combinations thereof
[0157] The base can be: DIEA (diisopropylethylamine), triethylamine, DMAP, pyridine or combinations thereof
[0158] (b) Under acidic conditions, the compound of formula II removes the ...
Embodiment 1
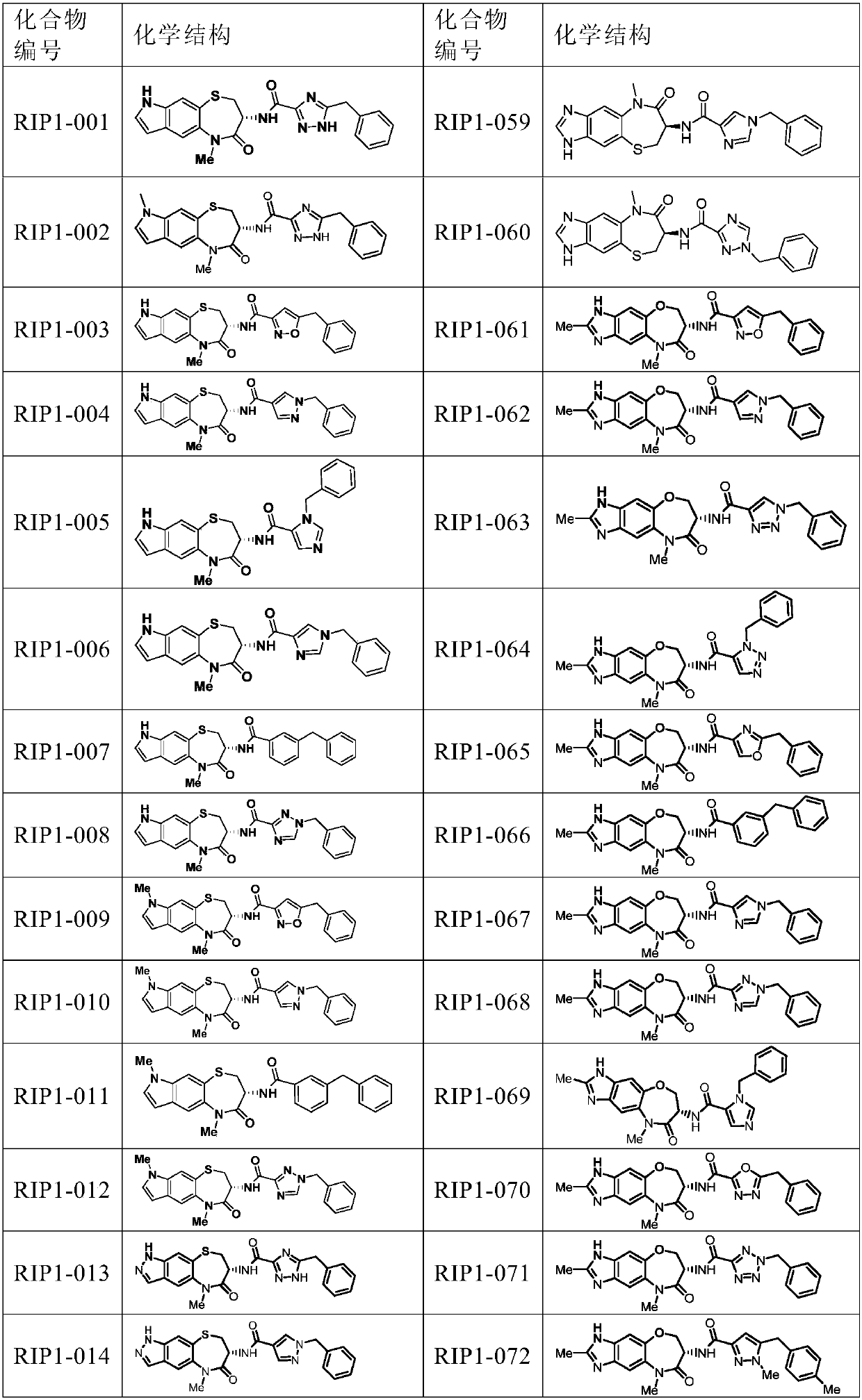
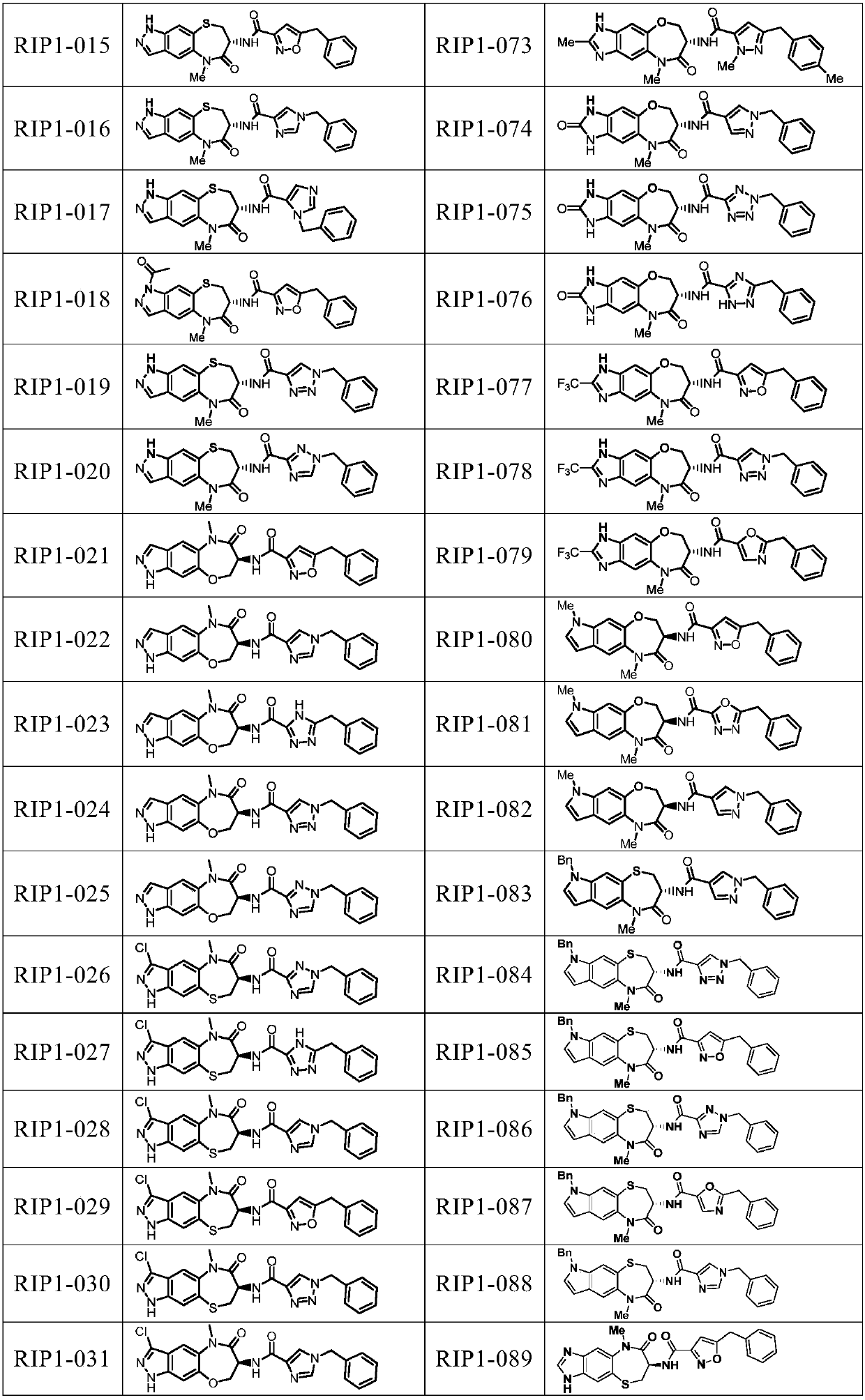
[0219] Embodiment 1, the preparation of condensation reaction synthetic compound I (RIP1-001-017, 019-0116)
[0220] method 1:
[0221]
[0222] Compound II-1 (27.8mg, 0.08mmol) was placed in a 25mL one-mouth bottle, 1mL TFA and 4mL DCM were added thereto, and the reaction was carried out at room temperature for 30min. After the reaction was monitored by TLC, the solvent was removed under reduced pressure, dried in vacuo and redissolved in 4mL DMF. Add HATU (38mg, 0.1mmol), DIEA (51.7mg, 0.4mmol), 5-benzyl-4H-1,2,4-triazole-3-carboxylic acid III-1 (20.3mg, 0.1mmol) to it, room temperature Reacted overnight, TLC monitored the end of the reaction, extracted with EA, washed with deionized water, washed with saturated NaCl, Na 2 SO 4 Dry, remove solvent under reduced pressure, reverse phase column separation and lyophilize to give RIP1-001: white solid 14.0 mg (40.4%)
[0223] By changing compound II and compound III (both known compounds, prepared by methods reported in the...
Embodiment 2
[0471] Embodiment 2, the synthesis of compound RIP1-018
[0472]
[0473] Compound RIP1-015 (13mg, 0.03mmol) was placed in a 25mL single-necked bottle, and 5mL of DCM, one drop of acetic anhydride, and two drops of TEA were added to it, and the reaction was carried out at room temperature for 30min. After the reaction was monitored by TLC, the solvent was removed under reduced pressure, extracted with EA, and deionized. Washed with water, washed with saturated NaCl, Na 2 SO 4 Drying, removal of solvent under reduced pressure, reverse phase column separation and lyophilization gave RIP1-018: white solid 10 mg (70.7%).
[0474] RIP1-018: 1 H NMR (400MHz, CDCl 3 )δ8.87(s, 1H), 8.17(s, 1H), 7.86(d, J=7.5Hz, 1H), 7.70(s, 1H), 7.38-7.29(m, 3H), 7.27-7.23(m , 2H), 6.32(s, 1H), 4.82-4.72(m, 1H), 4.12(s, 2H), 3.86(dd, J=11.1, 6.8Hz, 1H), 3.51(s, 3H), 3.01- 2.94(m, 1H), 2.85(s, 3H); 13 CNMR (100MHz, CDCl 3 )δ 174.1, 171.0, 169.7, 158.2, 158.1, 142.3, 139.1, 137.4, 135.3, 129.8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com