Preparation and application of alkaline phosphatase activated photosensitizer
A technology of photosensitizer and phosphatase, which is applied in medical preparations containing active ingredients, photodynamic therapy, compounds of Group 5/15 elements of the periodic table, etc., can solve side effects, prolong skin photosensitization, low selectivity, etc. problem, to achieve the effect of avoiding self-absorption, improving photodynamic ability and improving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
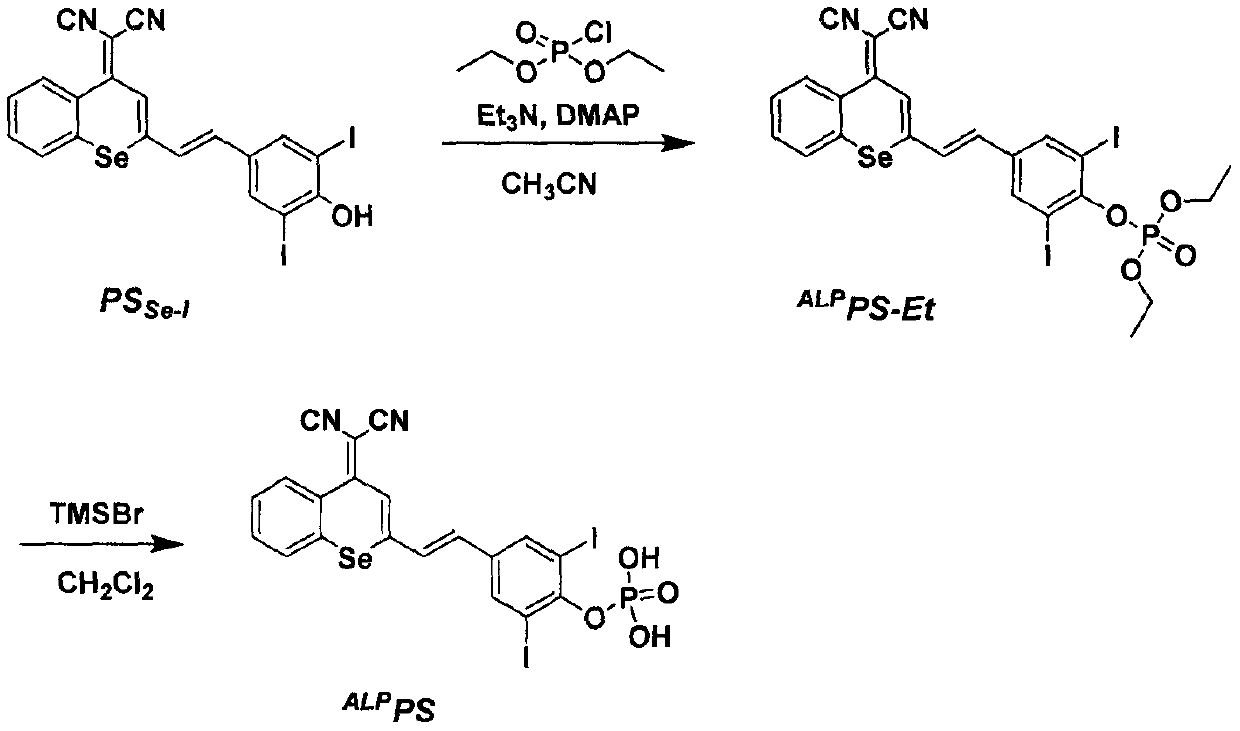
[0024] Embodiment one: synthesis ALP PS-Et
[0025] P.S. Se-I (0.5g, 0.80mmol) was dissolved in acetonitrile (20mL), diethyl chlorophosphate (0.21g, 1.22mmol) and triethylamine (0.12g, 1.2mmol) were added dropwise, and the reaction was stirred at room temperature for 5min Afterwards, DMAP (9.8mg, 0.08mmol) was added and stirring was continued overnight at room temperature. After the reaction, the solvent was removed by rotary evaporation, and purified by column chromatography using dichloromethane and methanol as eluents to obtain a bright red solid compound ALP PS-Et (0.2 g, 32.8%). 1 H NMR (400MHz, CDCl 3 ) δ (ppm) 8.76-8.67 (m, 1H), 7.99 (s, 2H), 7.78-7.68 (m, 2H), 7.61-7.51 (m, 2H), 7.11 (d, J = 15.6Hz, 1H) , 6.88(d, J=16.0Hz, 1H), 4.43-4.27(m, 4H), 1.42(t, J=7.2Hz, 6H). 13 C NMR (100MHz, CDCl 3 )δ(ppm)158.51,152.63,147.51,139.23,135.27,133.40,133.29,131.90,129.65,129.45,129.29,128.38,126.82,124.77,116.23,115.03,89.52,65.45,65.39,16.18, 16.11.
Embodiment 2
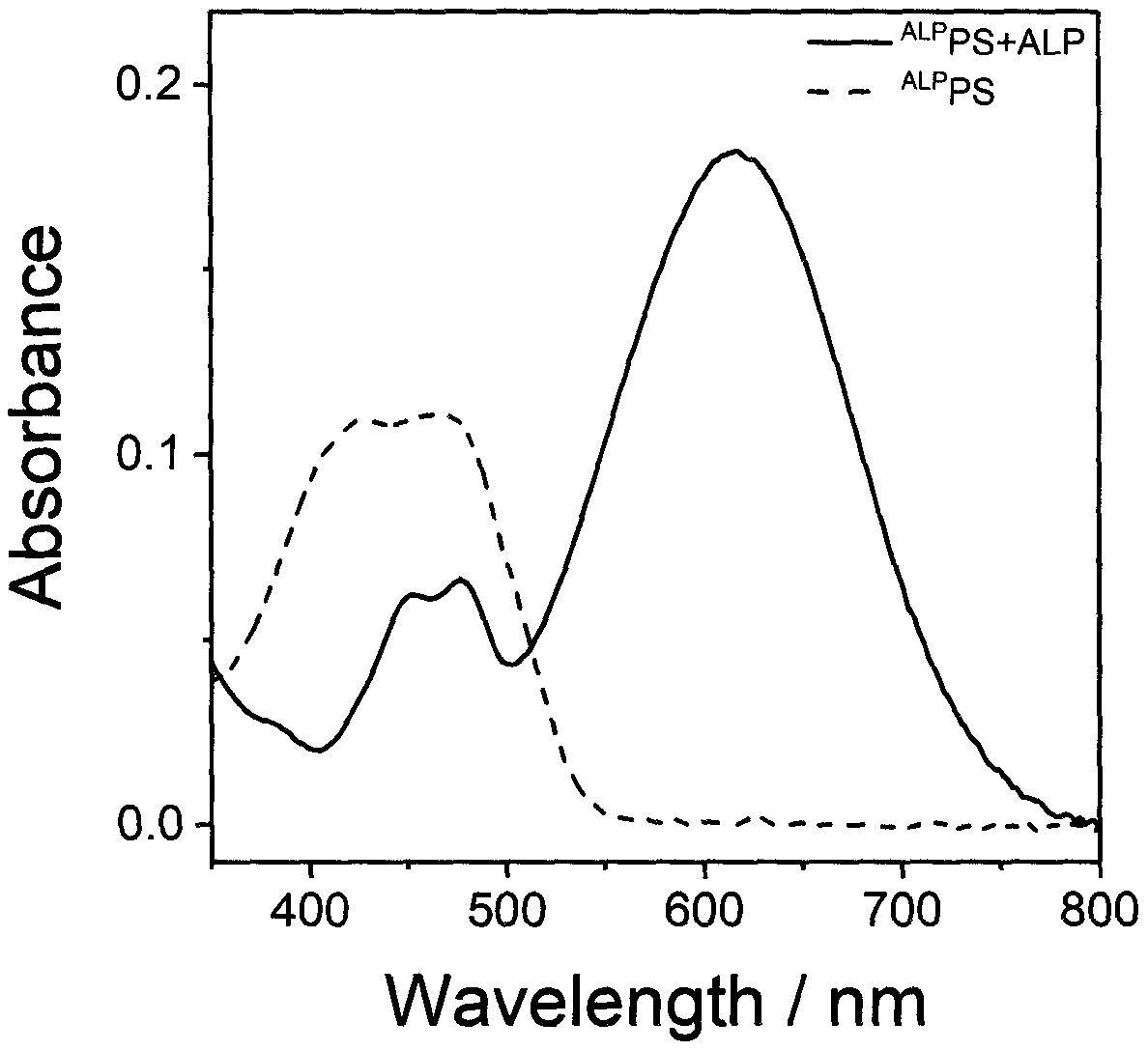
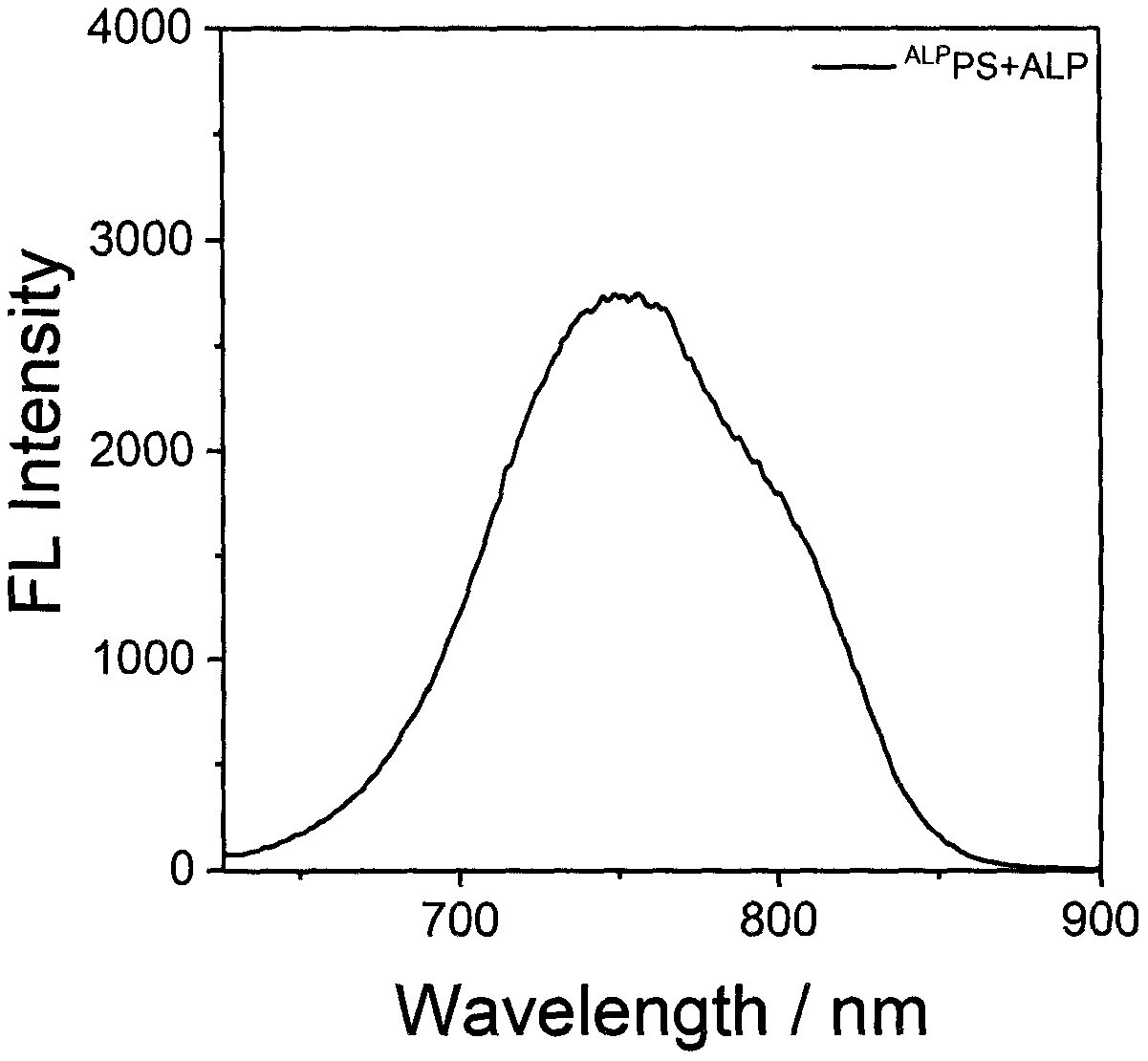
[0026] Embodiment two: synthesis ALP P.S.
[0027] ALP PS-Et (0.2g, 0.26mmol) was dissolved in dichloromethane (10mL), trimethylbromosilane (1mL) was slowly added dropwise under nitrogen protection, and reacted at room temperature for 8h. Filtration, dichloromethane (50mL) rinse filter residue, dry in a vacuum oven to obtain a red solid compound ALP PS (77.8 mg, 42.3%). 1 H NMR (400MHz, DMSO-d 6 ( s, 1H), 7.69 (m, 2H), 7.21 (d, J=16.0Hz, 1H). 13 C NMR (100MHz, DMSO-d 6 )δ (ppm) 158.98, 150.55, 139.28, 134.74, 134.19, 132.64, 130.60, 129.63, 128.63, 126.31, 124.22, 117.44, 115.91, 92.31, 71.98. HRMS (ESI): m / z [M+NH + ]calcd for C 20 h 11 I 2 N 3 OSe + 725.8049; found 725.8030.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com