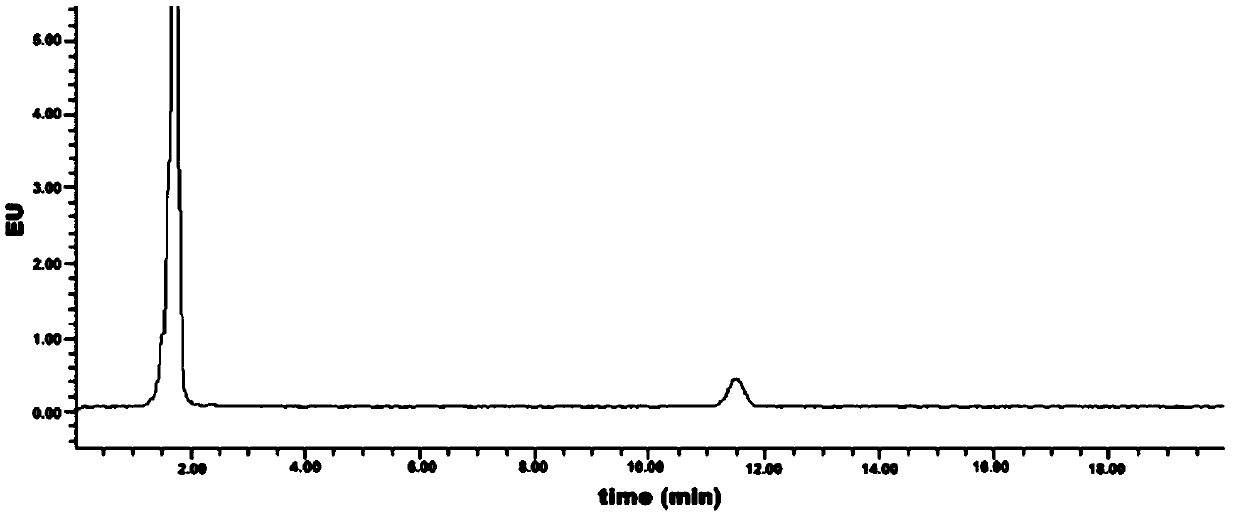
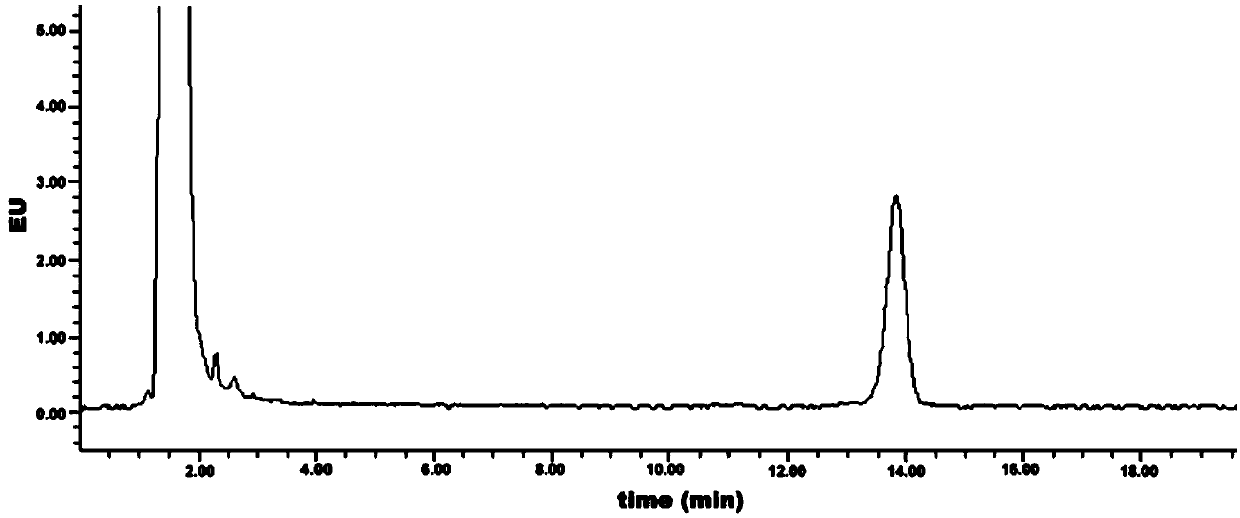
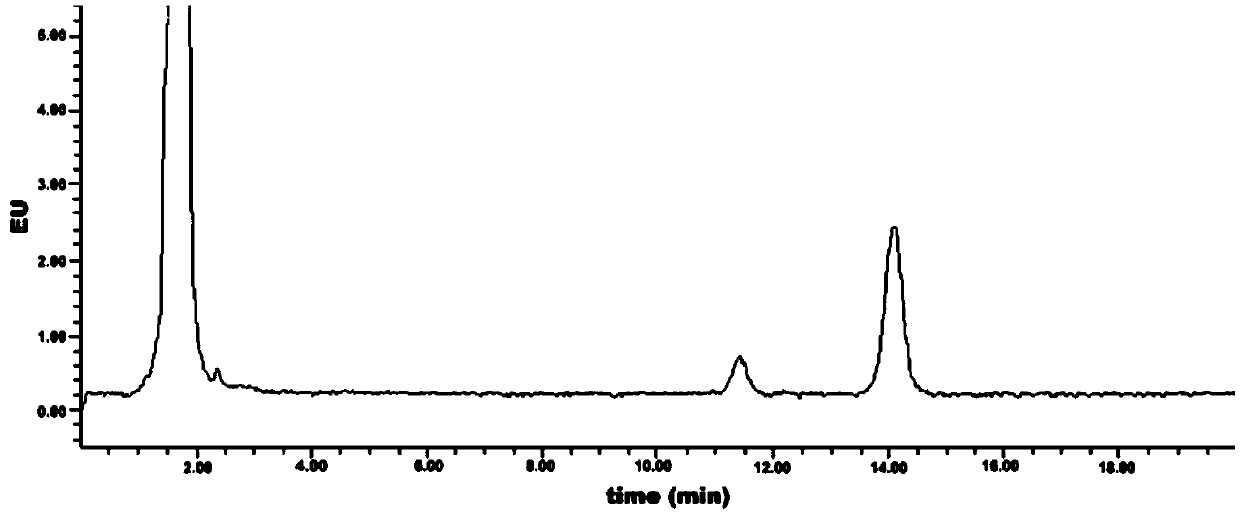
Method for detecting content of geranyl pyrophosphate based on prenylation reaction
A geranyl pyrophosphate reaction technology, applied in the field of pathophysiology and metabolite detection, can solve the problems of poor accuracy and low reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Purification of plasma or tissue samples by liquid-liquid extraction
[0029] (1) Take 1 mL of plasma or tissue grinding solution and add it to a 10 mL Ep tube;
[0030] (2) Add 350 μL of Tris-HCl solution to the above liquid, and then add 13.5 μL of alkaline phosphatase inhibitor (100×);
[0031] (3) Put the mixture into a constant temperature water bath and incubate at 37°C for 40 minutes;
[0032](4) Take out the sample from the constant temperature water bath, add 7mL liquid-liquid extractant and mix, the liquid-liquid extractant is a mixture of n-hexane and absolute ethanol, mix;
[0033] (5) Place the Ep tube on a shaker for 5 minutes, then put it into a bench-top low-speed centrifuge, centrifuge at 2000 rpm for 5 minutes;
[0034] (6) After centrifugation, the solution appears to be stratified. Take the organic layer, that is, the upper layer solution, and put it into a new 4mL Ep tube. If it cannot fit, divide it into two tubes and store it at 4°C f...
Embodiment 2
[0038] Example 2: Prenylation reaction to obtain the target compound and internal standard to be detected
[0039] (1) Take 48.5 μL sample and put it on ice, add 1 μL polypeptide solution (Dansyl-GCVLL), then add 0.5 μL LGTase (prenylation specific enzyme), mix well, the reaction solution is the reaction solution of the target compound;
[0040] (2) Take 48.5 μL of 2000ng / mL pure geranyl pyrophosphate and place it on ice, add 1 μL of polypeptide solution (Dansyl-CVIF), then add 0.5 μL of GGTase (specific prenylation reaction enzyme), mix well , the reaction solution is the reaction solution of the internal standard;
[0041] (3) Put the two groups of reaction solutions into a constant temperature water bath, react at 37°C for 90 minutes, take them out and place them on ice to terminate the reaction;
[0042] (4) Take 10 μL of the internal standard reaction solution and add it to the reaction solution of the target compound. After mixing, leave it for detection on the machine....
Embodiment 3
[0043] Embodiment 3: Measurement of the initial detection value of geranyl pyrophosphate
[0044] (1) High performance liquid chromatography detection
[0045] (1) Turn on the workstation, fluorescence detector, pump, column thermostat, sampling system and injector, and wait until the fluorescence detector is adjusted;
[0046] (2) Connect the C18 chromatographic column to the inside of the sampling system, pay attention to the outflow sequence of the chromatographic column, and close the column thermostat;
[0047] (3) Pour the pre-filtered aqueous mobile phase (ammonium acetate solution, pay attention to filter now) and organic phase mobile phase (chromatographic grade acetonitrile) into large-diameter solution bottles, and wrap the seal on the bottle mouth Membrane to prevent mobile phase volatilization;
[0048] (4) Detection conditions:
[0049]
[0050] (5) After setting the detection conditions on the workstation, wash with mobile phase for 30min-60min to balance ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com