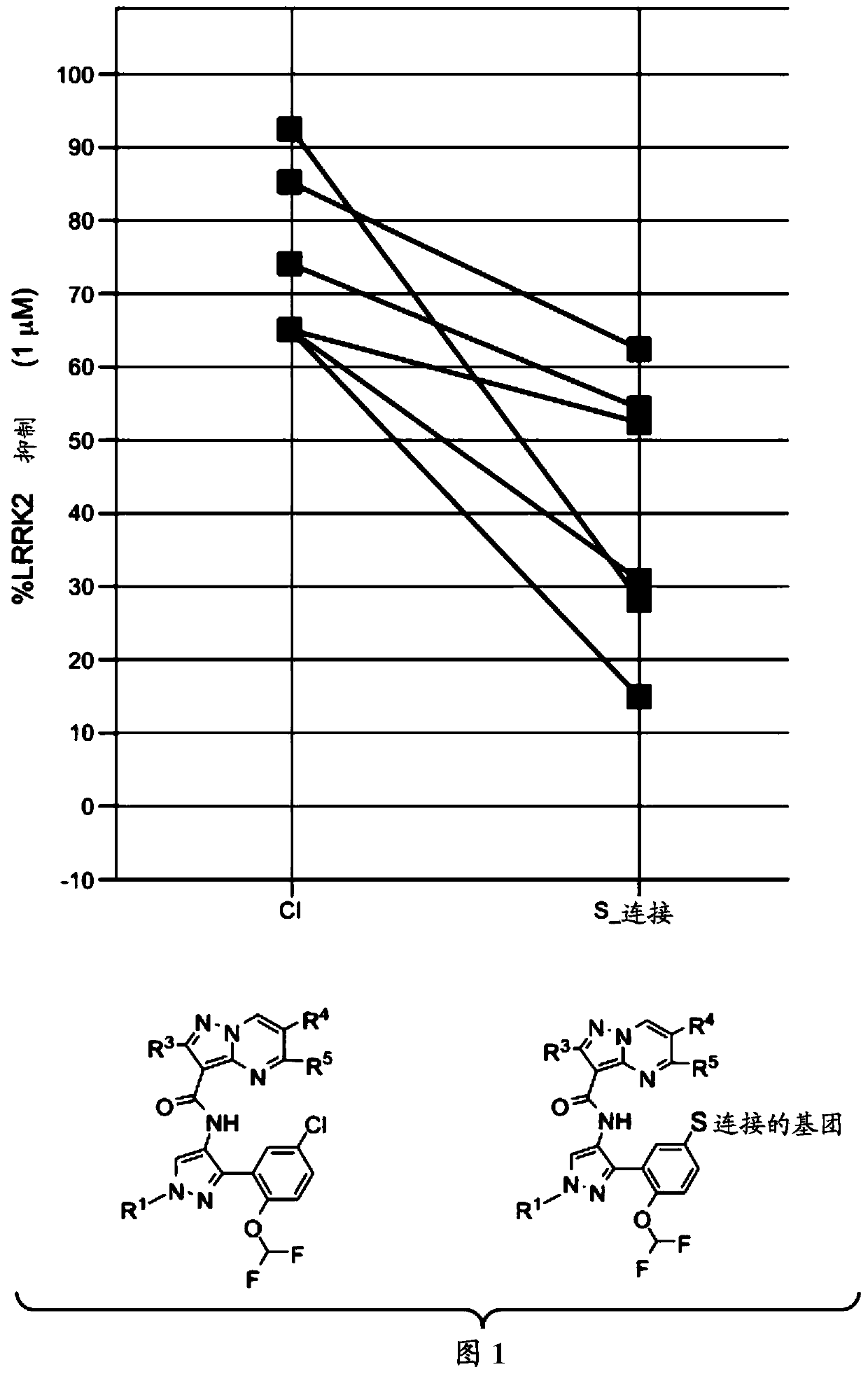
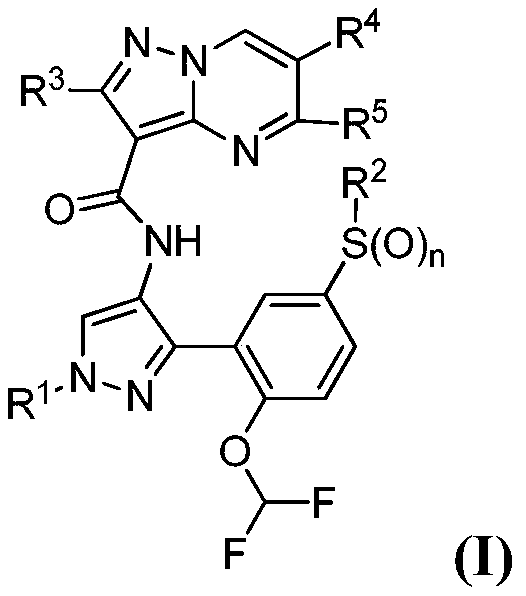
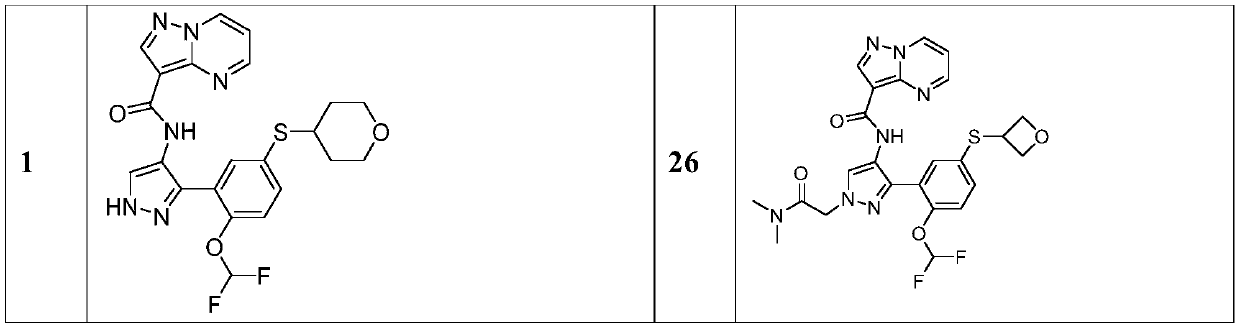
Therapeutic compounds and compositions, and methods of use thereof
A compound, alkyl technology, applied in the field of therapeutic compounds and compositions and their use, capable of solving problems such as defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0348] General Experimental Details
[0349] All solvents and commercially available reagents were used as received unless otherwise stated. When purifying the product by silica gel chromatography, use a glass column (Kieselgel 60, 220-440 mesh, 35-75 μm) manually packed with silica gel or SPE SiII column. 'Isolute SPE Si column' means a prepacked polypropylene column comprising unbonded reactive silica and an average size of 50 μm with a nominal porosity of irregular particles. when using SCX-2 column, SCX-2 column' means a pre-packed polypropylene column comprising a non-endcapped propanesulfonic acid functionalized silica gel strong cation exchange sorbent.
[0350] Procedure and LCMS conditions
[0351] Method A
[0352] Equipped with a C18 reversed-phase column (50x3mm Xtimate TM -C18, 2.2 μm particle size) SHIMADZU 20A HPLC, elution solvent A: water + 0.05% trifluoroacetic acid; solvent B: acetonitrile + 0.05% trifluoroacetic acid. gradient:
[0353]
[...
Embodiment 5
[0496]
[0497] N-(3-(2-(Difluoromethoxy)-5-((trifluoromethyl)sulfanyl)phenyl)-1H-pyrazol-4-yl)pyrazolo[1,5- a] pyrimidine-3-carboxamide
[0498] N-[5-[2-(difluoromethoxy)-5-[[tri(prop-2-yl)silyl]sulfanyl]phenyl]-1-[ [2-(Trimethylsilyl)ethoxy]methyl]-1H-pyrazol-4-yl]pyrazolo[1,5-a]pyrimidine-3-carboxamide (140mg, 0.203mmol) To a solution of TBAF (75 mg, 85%, 0.244 mmol) in DMA (1 mL) was added to a solution of DMA (2.5 mL). The mixture was stirred at 0 °C for 5 min, then 1-(trifluoromethyl)-3H-1-λ-3,2-benziodaoxol-3-one (85.0 mg, 0.269 mmol) in DMA (1 mL) was added dropwise . The mixture was allowed to warm to room temperature and stirred for 20 minutes. The reaction was repeated once on the same scale and the products of both reactions were combined for purification. The mixture was diluted with ethyl acetate (50 mL), washed with water (2x) and brine (2x), dried over anhydrous sodium sulfate and concentrated in vacuo. The residue was purified by flash chromatography ...
Embodiment 22
[0501]
[0502] N-(3-(2-(difluoromethoxy)-5-((1,3-difluoroprop-2-yl)sulfanyl)phenyl)-1-(2-hydroxyethyl)- 1H-pyrazol-4-yl)pyrazolo[1,5-a]pyrimidine-3-carboxamide
[0503] N-[3-[5-bromo-2-(difluoromethoxy)phenyl]-1H-pyrazol-4-yl]pyrazolo[1,5-a]pyrimidine-3-carboxamide (500mg, 1.11mmol), (2-bromoethoxy)(tert-butyl)dimethylsilane (292mg, 1.22mmol) and Cs 2 CO 3 (725 mg, 2.22 mmol) in N,N-dimethylformamide (15 mL) was heated at 60° C. under nitrogen for 2 hours and then allowed to cool to room temperature. The reaction mixture was partitioned between ethyl acetate (100 mL) and water (60 mL). The organic phase was washed with brine (2x), dried over anhydrous sodium sulfate and concentrated in vacuo. The residue was purified by flash chromatography on silica gel, eluting with ethyl acetate / petroleum ether (1 / 1). The appropriate fractions were combined and concentrated under reduced pressure to give 433 mg (64%) of N-[3-[5-bromo-2-(difluoromethoxy)phenyl]-1-[2-[(tert-butyldi ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com