Application of 27-hydroxycholesterol to preparation of product for diagnosing schizophrenia
A technology for hydroxycholesterol and schizophrenia, applied in the direction of disease diagnosis, analytical materials, material inspection products, etc., can solve the problems of unfavorable disease prognosis, lack of pathological diagnosis indicators, etc., and achieve convenient promotion and use, high sensitivity and specificity, and convenient The effect obtained
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1: the collection of peripheral blood and the method for measuring the concentration of peripheral blood 27OHC
[0059] 1. According to the experimental protocol and diagnostic criteria that have passed the ethical review, the healthy control population (HC), the genetically high risk of schizophrenia (first-degree relatives of the patient, FDR), the clinical ultra-high risk (UHR) and the patients with schizophrenia ( SCZ) 4 groups with a total of 312 people. The entry criteria for each group are as follows:
[0060] Healthy controls: using the Structured Clinical Interview for DSM Disorders-Fourth Edition (DSM-IV) clinical interview (Structured Clinical Interview for DSM-IV Axis I disorders-Patient Edition, SCID-I / P ) and SIPS screening showed no mental illness and no family history of mental illness.
[0061] Inclusion criteria for patients with schizophrenia: in line with the diagnostic criteria of the fourth edition of the American Diagnostic and Statis...
Embodiment 2
[0074] Example 2: Peripheral blood 24OHC or 27OHC is used as a biomarker for the diagnosis of schizophrenia
[0075] 1. A total of 312 gender-matched subjects were included in this study. The basic demographic information and patient symptom assessment of each group are shown in Table 2.
[0076] Table 2: Basic demographics of subjects and evaluation of clinical symptoms of patients
[0077]
[0078] Note: HC: healthy controls; FDR: first-degree relatives of schizophrenia; UHR: clinical ultra-high risk of schizophrenia; SCZ: patients with schizophrenia; SIPs: schizophrenia risk syndrome structured interviews; PANSS: positive and negative symptoms scale; P: positive symptoms; N: negative symptoms; G: general psychotic symptoms: T: total score; NA: missing value.
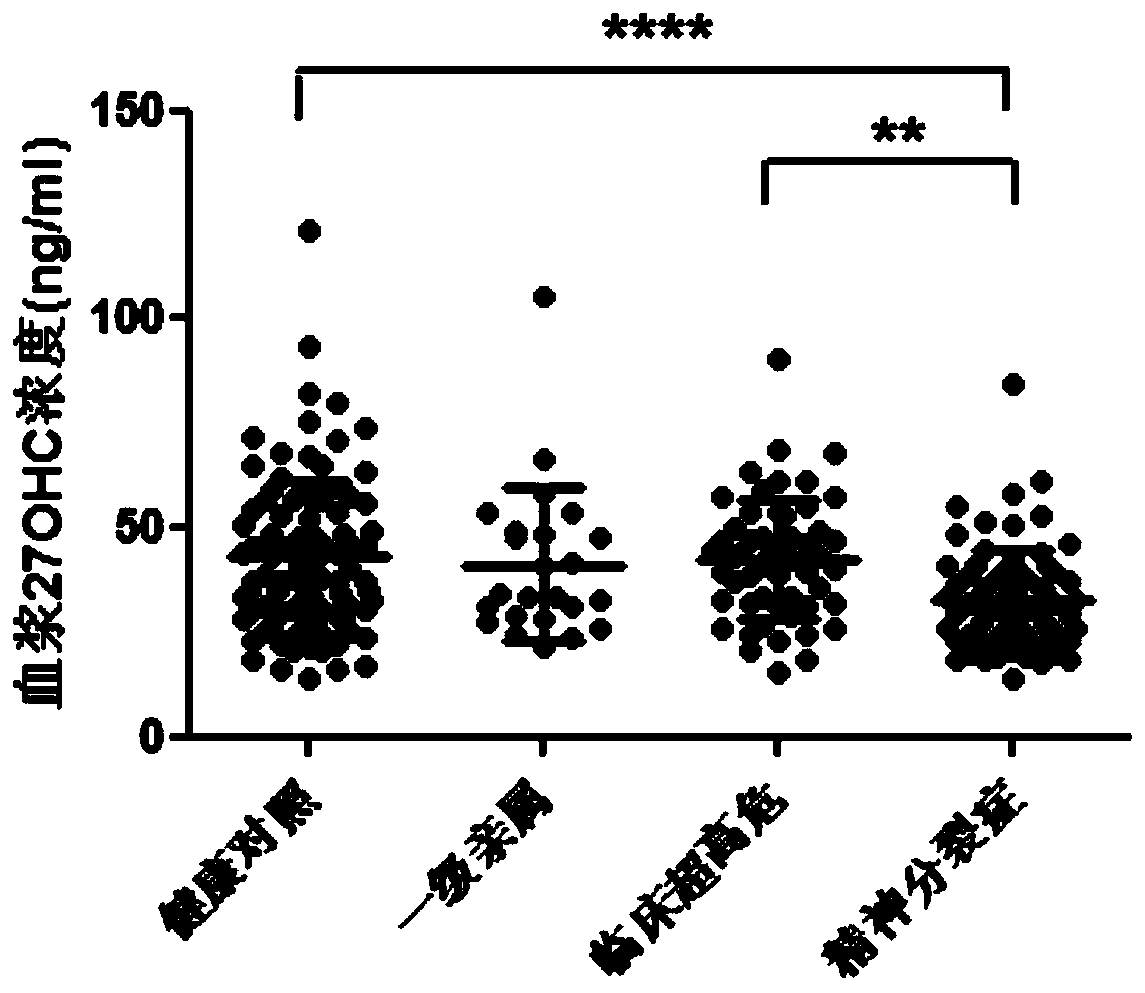
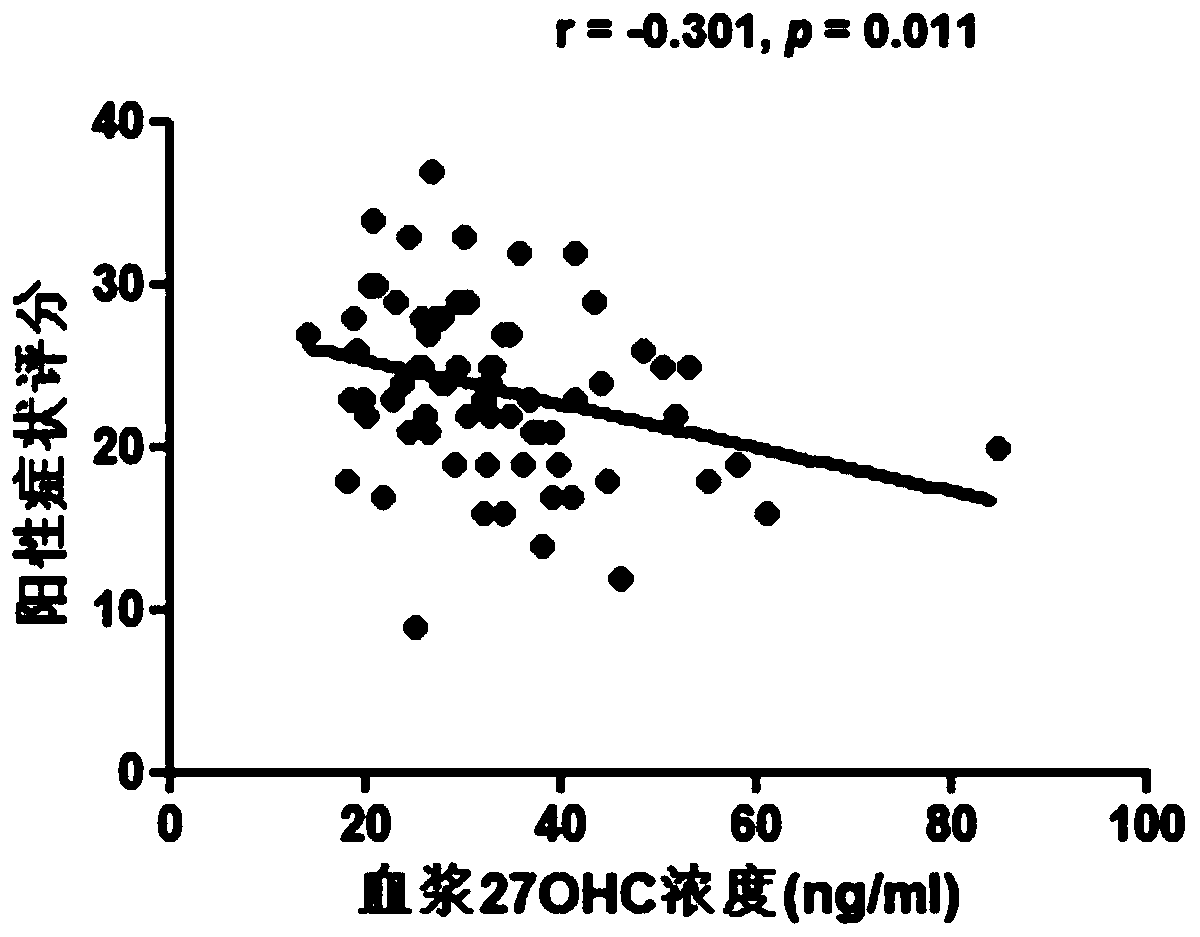
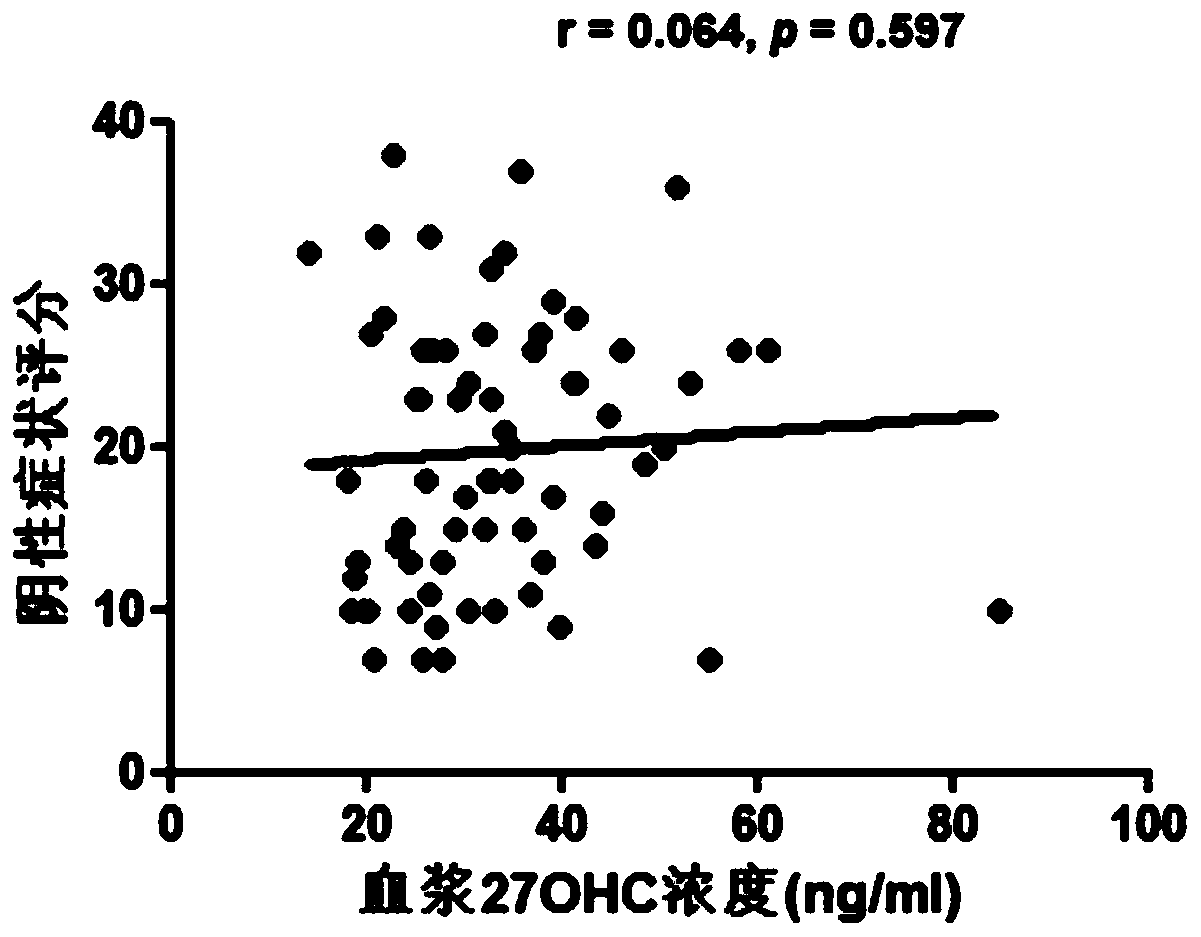
[0079] After the detection of plasma 27OHC, it was found that compared with the healthy control group, the plasma 27OHC level of patients with schizophrenia was significantly lower ( figure 1 , pfigure 1 ), indic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com