Serum amyloid protein A mutant as well as application and preparation method thereof
A protein mutant, serum starch technology, applied in chemical instruments and methods, botanical equipment and methods, biochemical equipment and methods, etc., can solve the limitation of SAA application, it is not easy to obtain SAA protein with natural structure, and stability is not stable. Good in vitro stability, improve in vitro stability, and retain the effect of antigenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Design of the amino acid sequence of the SAA mutant:
[0025] According to the amino acid sequence of the SAA protein published on the NCBI website and the characteristics of the sequence, the 52nd amino acid was replaced by Ala with Val, the 57th amino acid was replaced by Val with Ala, the 60th amino acid was replaced by Asp with Asn, and the 71st amino acid was replaced by Asp. The amino acid was replaced by Arg by His. Under the premise of retaining the antigenicity of natural SAA, the amino acid sequence of the obtained SAA mutant is shown in SEQ ID NO.1. The stability of this mutant is significantly better than that of native serum amyloid A.
Embodiment 2
[0027] Optimization and acquisition of SAA mutant gene sequence:
[0028] The nucleotide sequence of the SAA mutant was artificially optimized using the dominant codons of Escherichia coli, and finally the nucleotide sequence shown in SEQ ID NO.2 was obtained. The SAA mutant gene composed of dominant codons of Escherichia coli is obtained by annealing extension PCR technology, and the specific operation steps can refer to the Chinese invention patent with the authorized announcement number CN 104610443B.
Embodiment 3
[0030] Construction of SAA mutant expression vector:
[0031] The SAA mutant gene sequence obtained in Example 2 was double-digested with NdeI and XhoI and replaced between XhoI / NdeI of the prokaryotic expression vector pET-32a (+) (according to Merck Millipore, catalog number 69015) The fragment of pET-32a(+)-SAA (mutant type) was obtained.
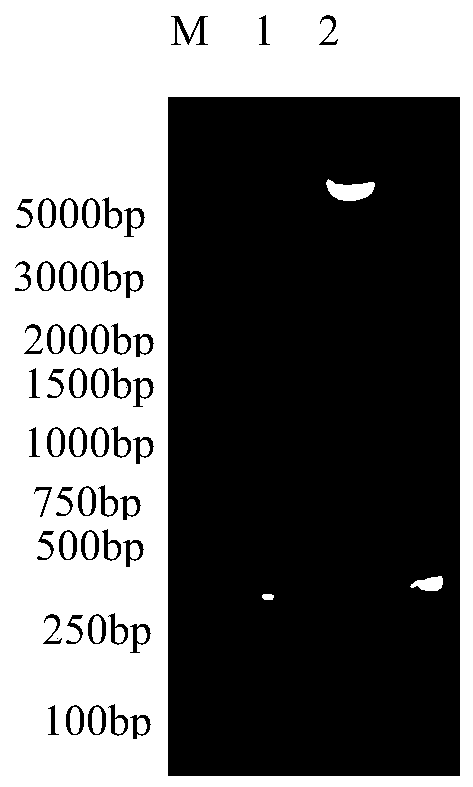
[0032] Recombinant plasmid pET-32a(+)-SAA mutant), pET-32a(+) and SAA mutant gene were identified by XhoI and NdeI double enzyme digestion, the results are as follows figure 1 As shown, where M: DL5000Marker; 1: pET-32a(+)-SAA (mutant) recombinant plasmid double digestion; 2: pET-32a(+) plasmid double digestion; 3; SAA mutant gene double digestion . Depend on figure 1 It can be seen that after the recombinant plasmid is digested with Xho I and Nhe I, two bands with the expected molecular weight can be seen in electrophoresis, which is consistent with the size of pET-32a(+) and the SAA mutant gene.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com