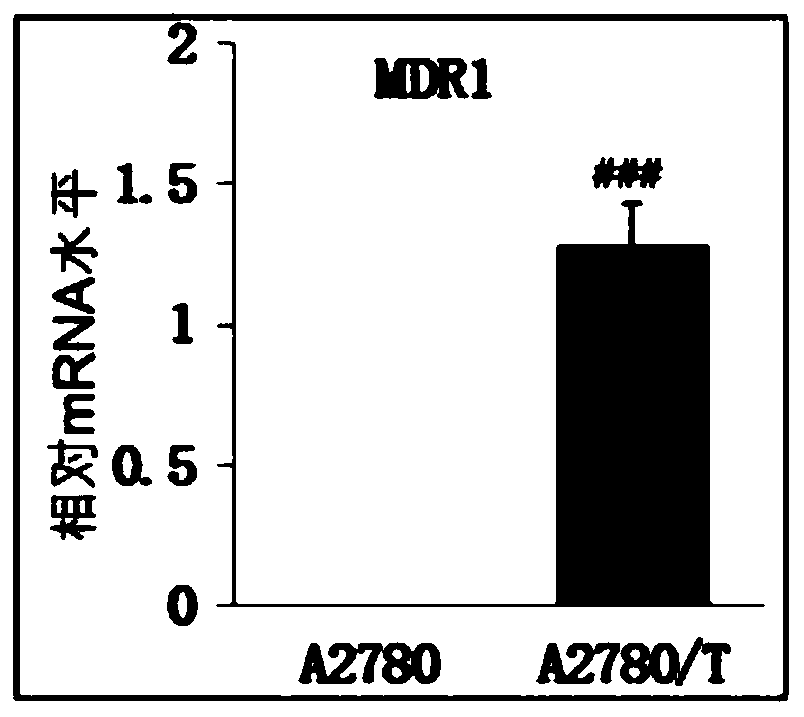
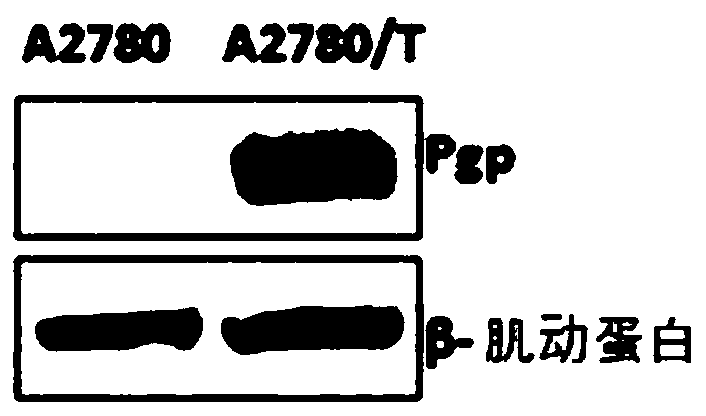
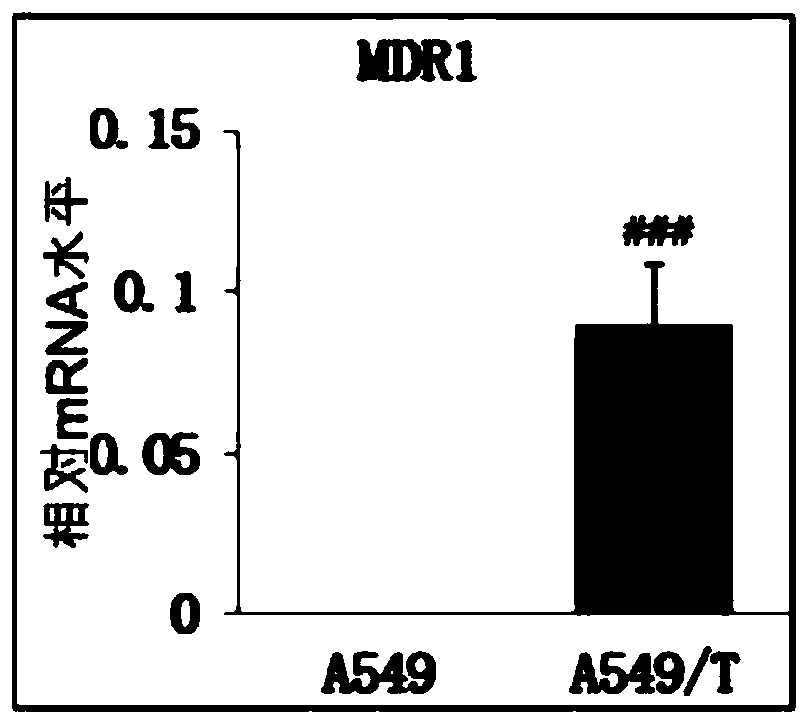
Application of compounds in preparation of drugs for treating multi-drug resistant cancers
A multi-drug resistance and chemotherapy drug technology, applied in the direction of drug combination, anti-tumor drugs, pharmaceutical formulations, etc., can solve the problems of unsuccessful multi-drug resistant tumor reversal agents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0115] Formula (II) compound cytotoxicity assay
[0116] The sulforhodamine B (SRB) assay is used to evaluate drug-induced cytotoxicity and cell proliferation. In short, before treatment, cells were collected, counted and planted on 96-well plates. Different concentrations of chemotherapeutics and Rg5 (concentrations 8, 4 and 2μM) were used to evaluate whether the combination can enhance the growth inhibitory effect of multidrug resistant cancer cells. Add 50 μL of culture medium containing 50% (W / V) TCA (10% final concentration of TCA), and incubate at 4° C. for 1 hour. The cells were washed five times with water, stained with 0.4% SRB in 1% V / V acetic acid solution (100 μl / well) for 10 minutes, then quickly washed with 1% acetic acid, and then dissolved with 200 μl of 10 mM Tris alkali solution (pH = 10.5 ). Evaluation of binding protein staining at a wavelength of 515nm ( Compound mode detection platform, molecular equipment, California, USA). By comparing the IC of chemo...
example 2
[0128] Effect of compound of formula (II) on apoptosis of multidrug resistant cancer cells
[0129] Cell cycle and apoptosis analysis
[0130] Flow cytometry is used to determine the effect of compounds of formula (II) on the cell cycle and apoptosis in multidrug resistance ABC transporter-dependent cancer cells. In the cell cycle experiment, A2780 / T cells were collected after 24 hours, 48 hours or 72 hours of treatment, and washed twice with ice-cold PBS. Add 70% ice-cold ethanol at 4°C overnight or at -20°C for 2 hours to fix the cells. After washing again with PBS, the cells were stained with a staining solution for 30 minutes at room temperature. This staining solution contained PI (50 μg / ml) and RNase (200 μg / ml). Then pellet the cells, wash and resuspend in PBS, the final cell concentration is 1×10 6 / ml. The cell fluid was analyzed by the flow cytometer BDFACS Aria (San Jose, CA).
[0131] In the apoptosis analysis experiment, 1×106 treated cells were collected, washed a...
example 3
[0135] Synergistic effect of formula (II) compound and chemotherapeutics
[0136] The Chou-Talalay method (1997: Cambridge (UK): Biosoft.) was used to evaluate the synergistic therapeutic effect of Rg5 and docetaxel. This method is used to calculate the "combination index" (CI), which quantitatively describes the synergy (CI) 1). In short, the drug-resistant A2780 / T cells were exposed to the serially diluted Rg5 and docetaxel mixture solution for 48 hours. At several concentration points higher and lower than twice the IC50 series concentration, the method described above is used to evaluate the cytotoxicity of the combined application. By using CalcuSyn software v.2.1 (Bio-soft), the synergy is further refined into synergy (CI=0.3-0.7), strong synergy (CI=0.1-0.3) and very strong synergy (CI <0.1).
[0137] The degree of cell death reaches 50% (ED 50 ) And 90% (ED 90 ), the combination index (CI) values of Rg5 and docetaxel are 0.23 and 0.05, as shown in Table 2. This indica...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com