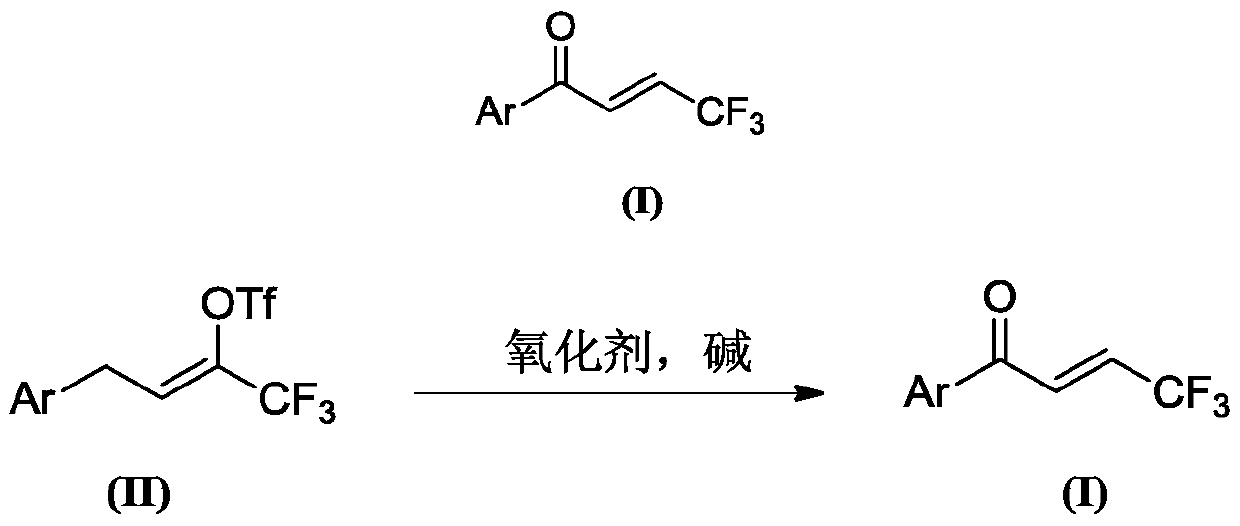
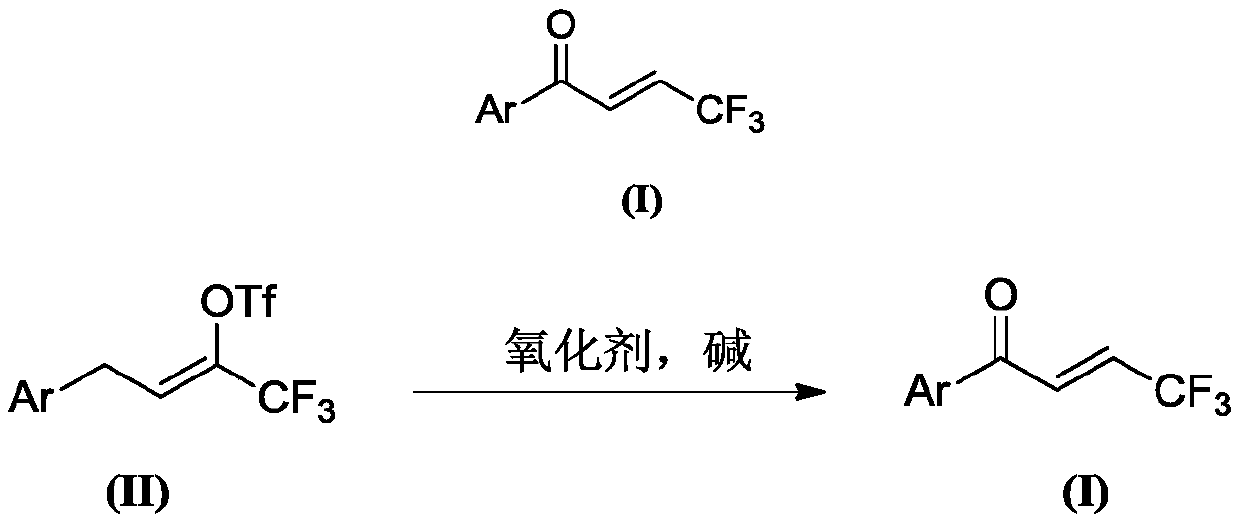
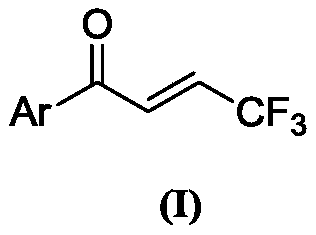
Preparation method of (E)-1-aryl-4, 4, 4-trifluorobutyl-2-ene-1-one compound
A technology of ketone compound and trifluorobutane, which is applied in the field of compound preparation, can solve problems such as limited application, expensive reaction conditions, and harshness, and achieve low-cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Preparation of (E)-1-phenyl-4,4,4-trifluorobut-2-en-1-one (compound 1)
[0056] At room temperature, add 334 mg (1.0 mmol) of 4-phenyl-2-trifluoromethanesulfonyl-1,1,1-trifluoro-2-butene, anhydrous solvent 4 mL of 1,2-dichloroethane, 4-methylpyridine-N- Oxide 218mg (2.0mmol), 4-phenyl-2-trifluoromethanesulfonate-1,1,1-trifluoro-2-butene 1.0 times molar amount of triethylamine 101mg (1.0mmol), placed React at 25°C for 3 hours. After the solvent was removed by rotary evaporation under reduced pressure, the target compound was obtained by column chromatography, the filler was silica gel, the eluent was petroleum ether, and the separation yield was 81%.
Embodiment 2
[0058] Preparation of (E)-1-(4-methylphenyl)-4,4,4-trifluorobut-2-en-1-one (compound 2)
[0059] In addition to replacing the 4-phenyl-2-trifluoromethanesulfonate-1,1,1-trifluoro-2-butene in Example 1 with the same molar amount of 4-(4-methylphenyl) Except for -2-trifluoromethanesulfonyl-1,1,1-trifluoro-2-butene, the same method as in Example 1 was followed to obtain the target compound with an isolated yield of 84%.
Embodiment 3
[0061] Preparation of (E)-1-(3-methylphenyl)-4,4,4-trifluorobut-2-en-1-one (compound 3)
[0062] In addition to changing the 4-phenyl-2-trifluoromethanesulfonate-1,1,1-trifluoro-2-butene in Example 1 to the same molar amount of 4-(3-methylphenyl) Except for -2-trifluoromethanesulfonyl-1,1,1-trifluoro-2-butene, the same method as in Example 1 was followed to obtain the target compound with an isolated yield of 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com