5-nitro orotic acid cobalt complex as well as preparation method and application thereof
A technology of nitroorotic acid and cobalt complexes, which is applied in the field of coordination chemistry, can solve problems such as the inability to explain the bonding and properties of coordination compounds, and achieve excellent physical and chemical properties and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Weigh CoCl 2 ·6H 2 O (0.238g, 1.0mmol), 5-nitroorotic acid (0.201g, 1.0mmol), 1,3-two (4-pyridine) propane (0.198g, 1.0mmol) were placed in a round bottom flask, and Distilled water 10mL, ethanol 5.0mL and DMF 1.0mL, stirred at room temperature for 10min, then transferred the mixture to a stainless steel reactor at 130°C for 48h, cooled naturally to room temperature, separated by filtration to obtain red blocky crystals.
Embodiment 2
[0025] Weigh Co(OAc) 2 4H 2 O (0.249g, 1.0mmol), 5-nitroorotic acid (0.201g, 1.0mmol), 1,3-two (4-pyridine) propane (0.198g, 1.0mmol) were placed in a round bottom flask, and Distilled water 10mL, ethanol 5.0mL and DMF 1.0mL, stirred at room temperature for 10min, then transferred the mixture to a stainless steel reactor at 160°C for 24h, cooled naturally to room temperature, separated by filtration to obtain red blocky crystals.
Embodiment 3
[0027] Weigh Co(NO 3 ) 2 ·6H 2 O (0.291g, 1.0mmol), 5-nitroorotic acid (0.201g, 1.0mmol), 1,3-bis(4-pyridine)propane (0.198g, 1.0mmol) were placed in a round bottom flask, added 15mL of distilled water, 7.5mL of ethanol and 1.5mL of DMF were stirred at room temperature for 10min, then the mixture was transferred to a stainless steel reactor at 140°C for 36h, cooled naturally to room temperature, separated by filtration to obtain red blocky crystals.
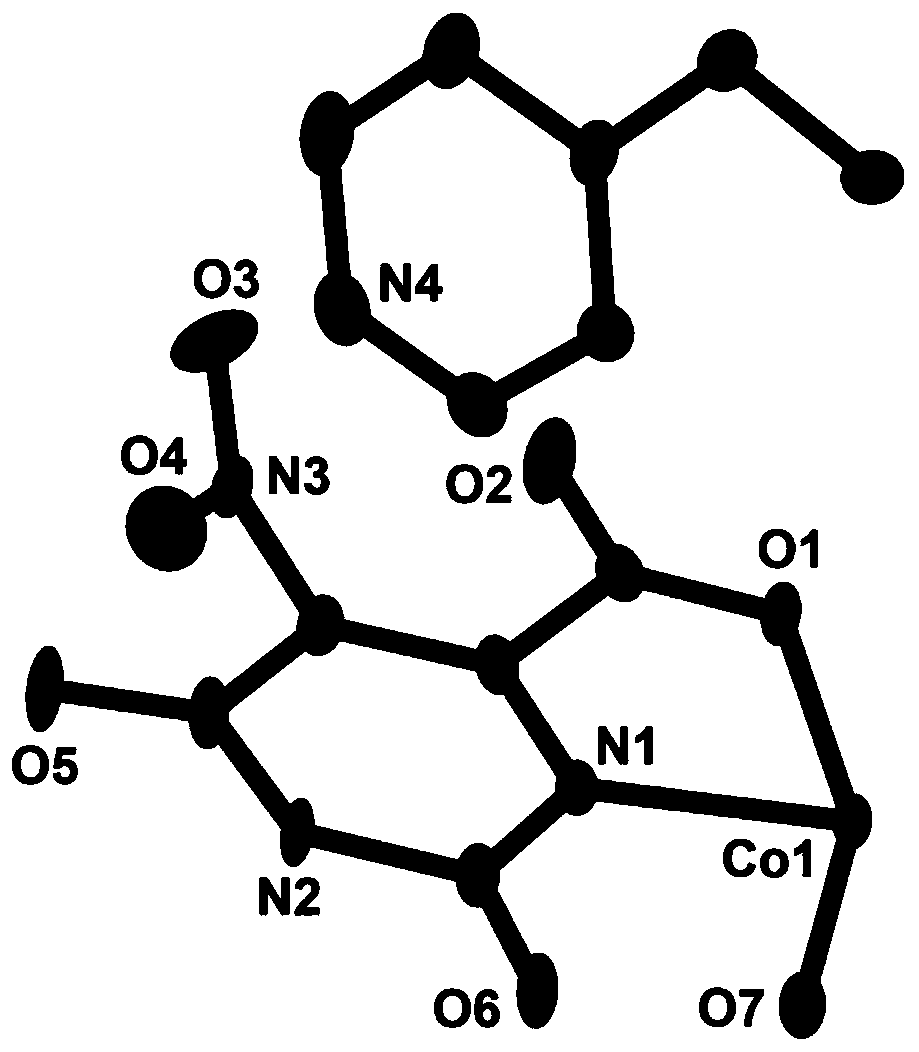
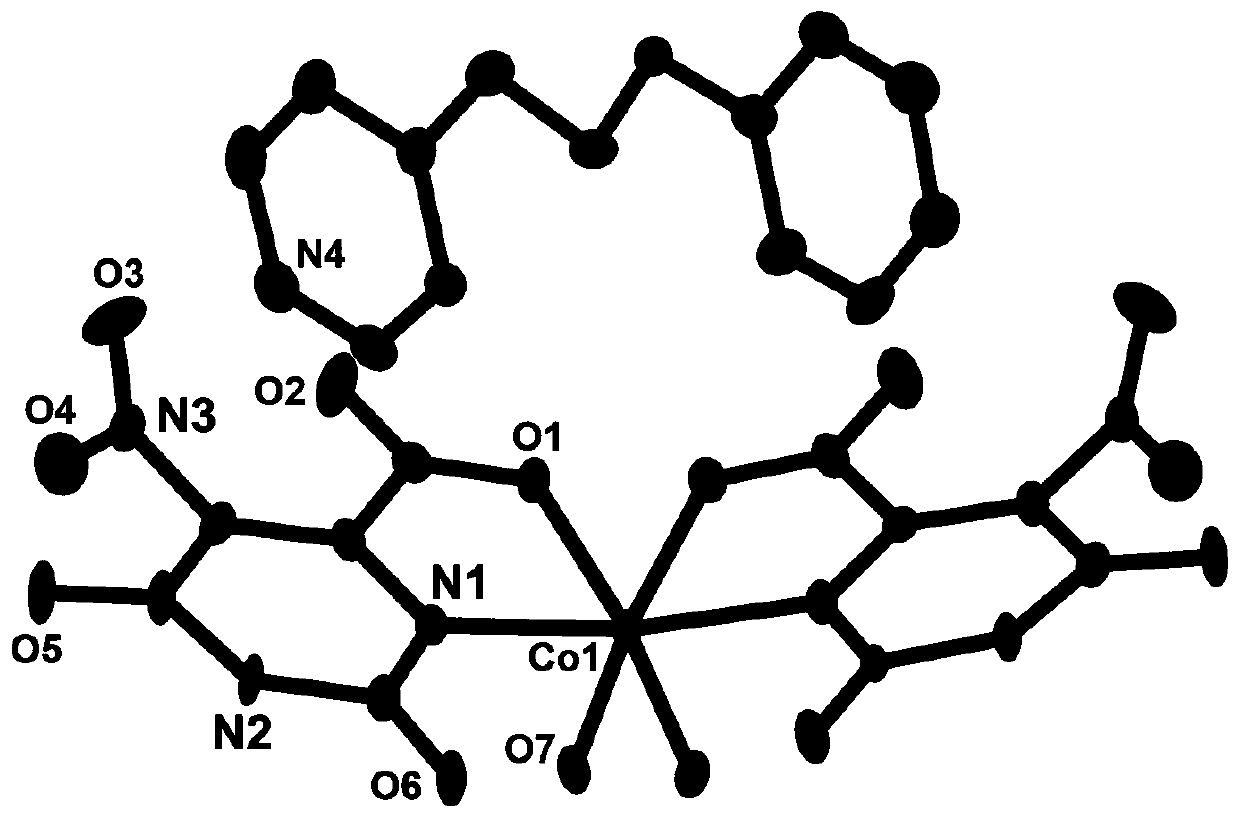
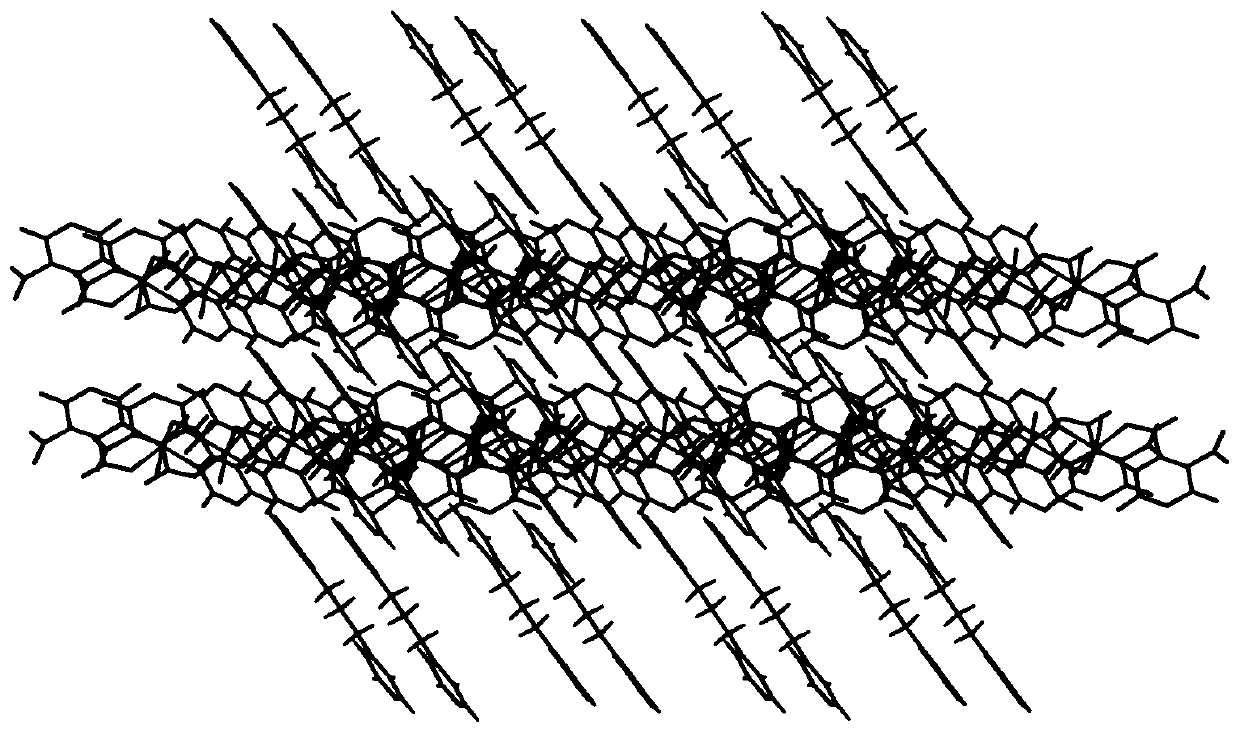
[0028] The red blocky crystals prepared in the above examples were subjected to X-ray single crystal diffraction test and analysis. It was determined that the occupancy ratios of cobalt ions, ligands and coordination water were all 0.5, and its asymmetric structural unit was composed of 0.5 cobalt ions , 0.5 5-nitroorotate, 0.5 1,3-bis(4-pyridine) propane, and 0.5 coordinated water molecules. The simplified structure is [Co 0.5 (HL) 0.5 (bpp) 0.5 (H 2 O) 0.5 ], the crystal system is triclinic, the space group is P-1, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com