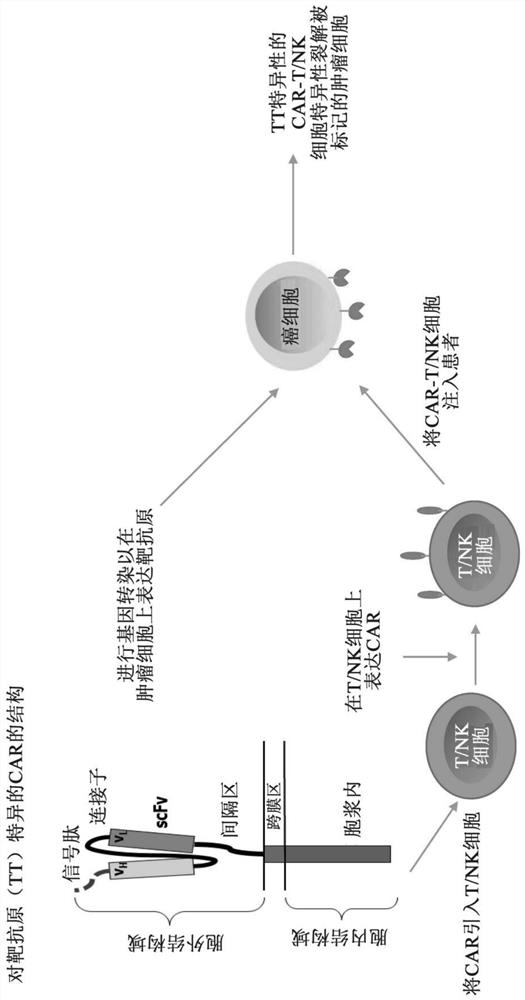
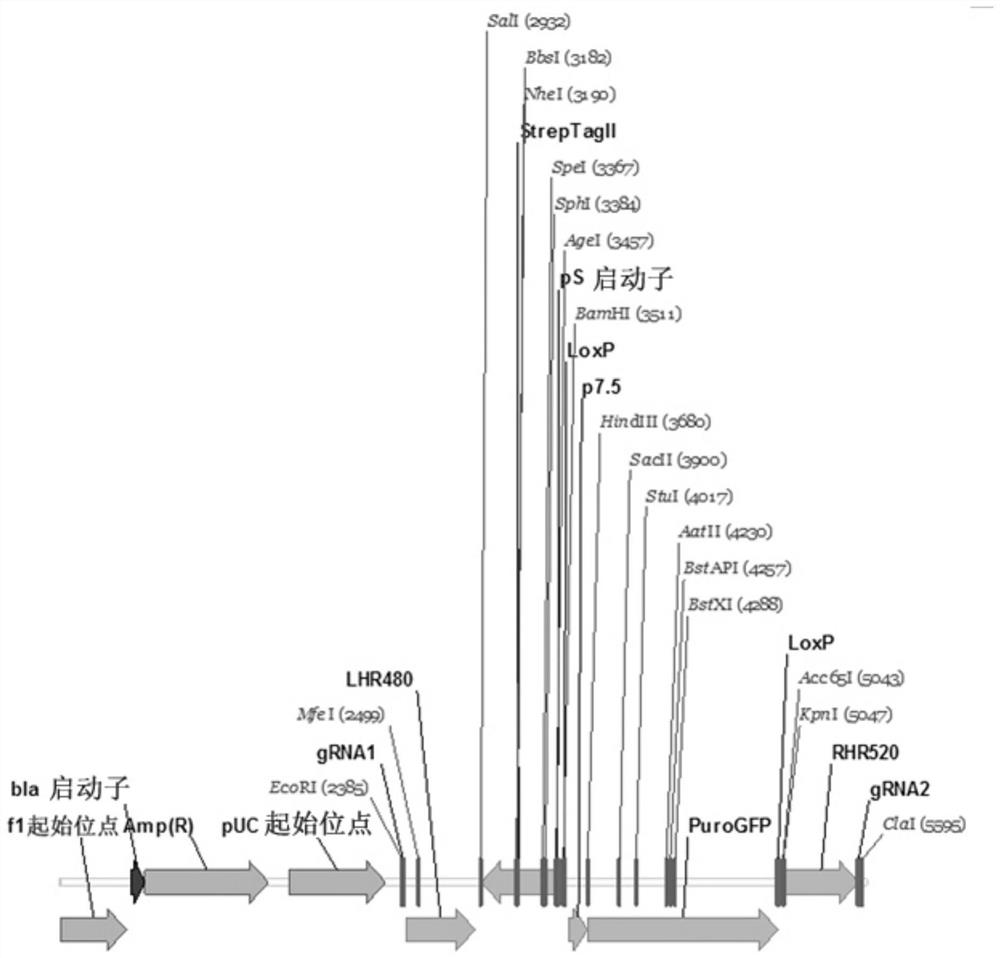
Therapeutic agent and application thereof comprising nucleic acid and car-modified immune cells
A therapeutic agent and tumor cell technology, applied in the field of medical bioengineering, can solve the problems of high risk of on-target/off-target toxicity, inability to exert the killing effect of cancer cells, poor T-cell tumor homing, etc. Effects of improved homing ability, reduced risk of on-target/off-target toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0321] Preparation Example 1: Preparation of tumor cells labeled with TT1, TT2, TT3, C1&2a, C1&2b by electroporation
[0322] Will 5x10 6 Jurkat cells, HCT116-luc, SKOV3-luc or SK-HEP-1 with 5 μg of TT1 (SEQ ID NO:11), TT2 (SEQ ID NO:12) or TT3 (SEQ ID NO:13), C1 & 2a (SEQ ID NO:11), respectively SEQ ID NO: 14), C1 & 2b (SEQ ID NO: 15) mRNAs (obtained according to the method of Preparation Example 8) were mixed in electroporation solution P3 (product name "P3Primary Cell 4D-X Kit L", Lonza, article number V4XP-3012), placed in a 100 μl Nucleocuvette™ tube (product name “P3Primary Cell 4D-X Kit L”, Lonza, Cat. No. V4XP-3012), and subjected to an ice bath for 5 minutes. Then use the 4D-NucleofectorTM electroporator (Lonza) to select its own tumor cell electroporation program for electroporation. After electroporation, the cells were removed and placed in the corresponding tumor cell culture medium. Jurkat medium is RPMI (Gibco)+10%FBS (Hyclone), SKOV3-luc and HCT116-luc mediu...
preparation example 2
[0326] Preparation Example 2: Preparation of CAR-T cells targeting TT1, TT2 and TT3 respectively
[0327] Will 2 x 10 6 Human PBMC cells were resuspended in 1 ml of T cell culture medium (AIMV (Gibco) supplemented with 1% human AB serum (Valley Biomedical)) supplemented with OKT3 (eBioscience) at a final concentration of 100 ng / ml and hrIL-2 at 300 IU / ml (Peprotech), seeded into one well of a 24-well plate at 37°C, 5% CO 2 The humidification cell incubator (Thermo Fisher). Supplement hrIL-2 with a final concentration of 300IU / ml every 2-3 days. According to the growth of cells, add fresh T cell culture medium and adjust the number of cells to 1×10 6 cells / ml.
[0328] T cells (1 × 10 7 ) and 4 μg aTT3-CD8-41BB-CD3ζ mRNA (that is, the mRNA corresponding to the nucleotide sequence shown in SEQ ID NO: 55 (obtained according to the method of Preparation Example 8)) mixed in electroporation solution P3 (product name “P3Primary Cell4D-X”) Kit L", Lonza, Cat. No. V4XP-3012), pla...
Embodiment 1
[0330] Example 1: Tumoricidal ability of CAR-T cells targeting marker polypeptides to tumor cells labeled with marker polypeptides by electroporation
[0331] This example tests the killing ability of CAR-T cells directed against the labeled polypeptides to Jurkat cells after electrotransduction. The CAR-modified or unmodified T cells targeting TT1 or TT2 obtained by the method of Preparation Example 2 were co-cultured with Jurkat, a human T cell line labeled with TT1 or TT2 by electroporation obtained by the method of Preparation Example 1, respectively. In a U-shaped 96-well plate, the ratio of the number of CAR-T effector cells to target cells (E:T) ranged from 1.25:1 to 20:1. Each group of experiments was repeated 3 times. After 2 hours of co-culture, DELFIA EuTDA Cytotoxicity Kit (PerkinElmer, USA) was used to detect the ability of CAR-T cells to lyse tumor cells, and the killing effect was calculated by the following formula: % specific lysis = ((experimental group rele...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com